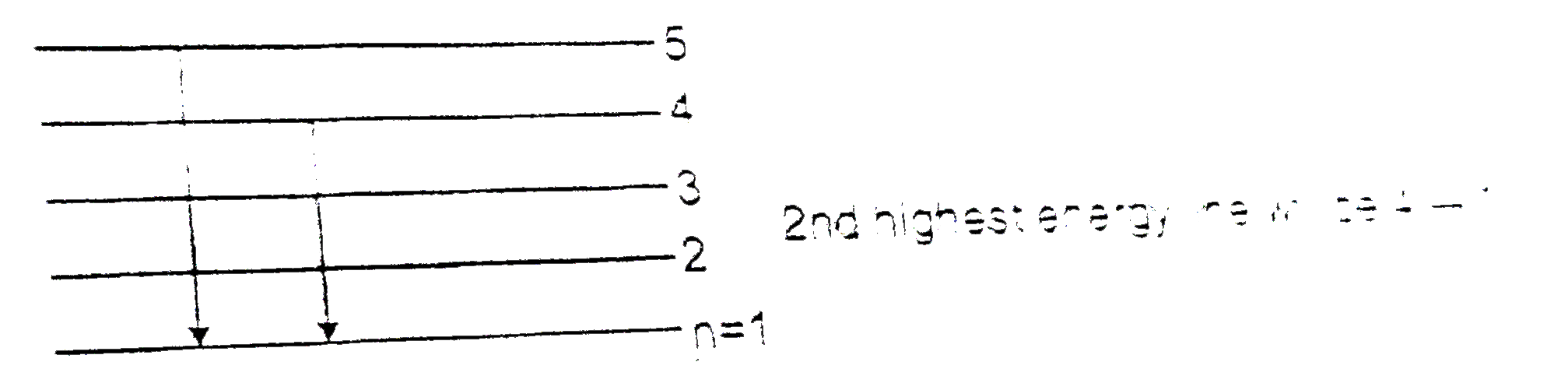Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-RANK BOOSTER-All Questions
- Electrons in a sample of H-atoms make transition from state n=x to som...
Text Solution
|
- A hydrogen like atom (atomic number z) is in a higher excited state of...
Text Solution
|
- In a sample of H-atoms in ground state electrons make transition from ...
Text Solution
|
- When a graph is plotted between log x//m and log p, it is striaght lin...
Text Solution
|
- Which of the following statements about physical adsorption is correct...
Text Solution
|
- Following is the variation of physical adsorption with temperature.
Text Solution
|
- Finely divided catalyst has greater surface area and has greater catal...
Text Solution
|
- Gold number of a lyphilic sol is such property that:
Text Solution
|
- For the coagulation of 200 mL of As(2)S(3) solution, 10 mL of 1 M NaCl...
Text Solution
|
- At CMC, the surfactant molecules :
Text Solution
|
- Which of the following ions will be most effective in coagulating the ...
Text Solution
|
- Coagulation value of the electrolytes AlCl(3) and NaCl for As(2)S(3) s...
Text Solution
|
- Which of the following is wrong :
Text Solution
|
- Gold number of haemoglobin is 0.03. Hence 100 ml of gold sol will requ...
Text Solution
|
- Oil-soluble dye is mixed with water-in-oil emulsion, then
Text Solution
|
- Which can absorb large volume of hydrogen gas ?
Text Solution
|
- The correct statement (S) pertaining to the adsorption of a gas on a s...
Text Solution
|
- In the aqueous solution of soaps above :
Text Solution
|
- Which of the following statements are true for physisoption?
Text Solution
|
- Which of the following are the correct :
Text Solution
|