Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-RANK BOOSTER-All Questions
- Match list I with list II and select the correct answer : {:("Column...
Text Solution
|
- Match list I (Colloidal system ) with list II (Example) {:("Column-I...
Text Solution
|
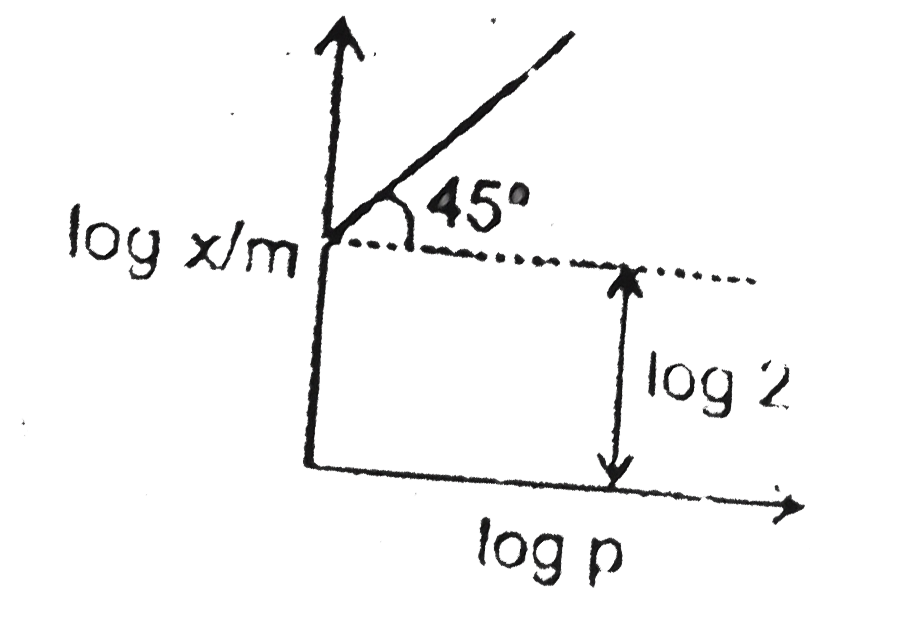
- At 2 atm pressure the value of x/m will be :(log 2=0.3010)
Text Solution
|
- 1 L of 0.6 M acetic acid is shaken with 2 g activated carbon.Activated...
Text Solution
|
- 100 ml of a colloidal solution is completely precipitated by addition ...
Text Solution
|
- On addition of the 1 ml. solution of 10% NaCl to 10ml gold sol in the ...
Text Solution
|
- In a coagulation experiment, 5mL of As(2)S(3) is mixed with distilled...
Text Solution
|
- The volume of nitrogen gas Um (measured at STP) required to cover a sa...
Text Solution
|
- The minimum concentration of an electrolyte required to cause coagulat...
Text Solution
|
- The electron gain enthalpies of halogens are as given below: F =- 33...
Text Solution
|
- Which of the following statements is wrong for the transition elements...
Text Solution
|
- If the same element is forming oxides in different oxidation states th...
Text Solution
|
- Which of the following represent the correct order of electron affinit...
Text Solution
|
- Assertion: In general, for an element, IE(1) lt IE(2) lt IE(3).... R...
Text Solution
|
- Statement-1: Third ionisation energy of phosphorous is larger than sul...
Text Solution
|
- Statement-1:Manganese (atomic number 25) has a less favourable electro...
Text Solution
|
- Assertion: Electron gain enthalpy always becomes less negative as we g...
Text Solution
|
- The periodicity is related to the electronic configuration. That is, a...
Text Solution
|
- The periodicity is related to the electronic configuration. That is, a...
Text Solution
|
- The main group elements complete their elctron configuration using s a...
Text Solution
|