A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-RANK BOOSTER-All Questions
- Which of the following are isoelectronic and isostructural ?
Text Solution
|
- Anhydrous Al Cl(3) is covalent . From the data given below Lattice E...
Text Solution
|
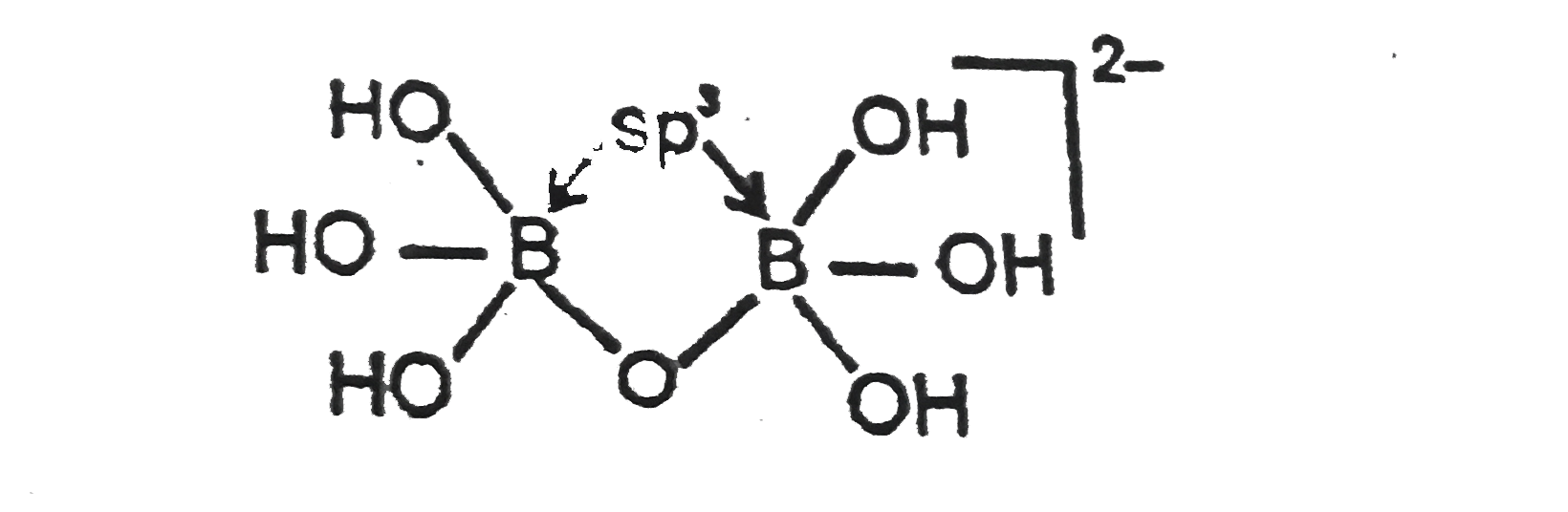
- What is the hybridisation of boron atoms in compound Mg[B2O(OH)6] ?
Text Solution
|
- Draw the structure of XeF4 molecule.
Text Solution
|
- Match the following and choose the correct option given below (a) N(...
Text Solution
|
- According to molecular orbital theory which of the following is correc...
Text Solution
|
- Which of the following statements are correct? (I) N(2)H(4) is pyra...
Text Solution
|
- Which charge for the N2 molecule would give a bond order of 2.5 ?
Text Solution
|
- Which of the following species is/are polar in nature ?
Text Solution
|
- Resonating structures have same :
Text Solution
|
- There is S-S linkage in :
Text Solution
|
- Which of the following contains odd electron bond ?
Text Solution
|
- In which pairs is are the stronger bond found in the first species ?
Text Solution
|
- Which of the following statement is/are true ?
Text Solution
|
- Hydrogen bonding is present in which of the following species ?
Text Solution
|
- Assertion : F bond angle p is equal to the bond angle Q but not preci...
Text Solution
|
- Assertion Bond order in a molecule can assume any value positive integ...
Text Solution
|
- In the valence bond theory, hybridisation of orbitals is an integral p...
Text Solution
|
- The distribution of electrons among molecular orbitals is called the e...
Text Solution
|
- {:("Column-I","Column-II"),((A)XeF4,(p)sp^3d "see-saw geometry"),((B)S...
Text Solution
|