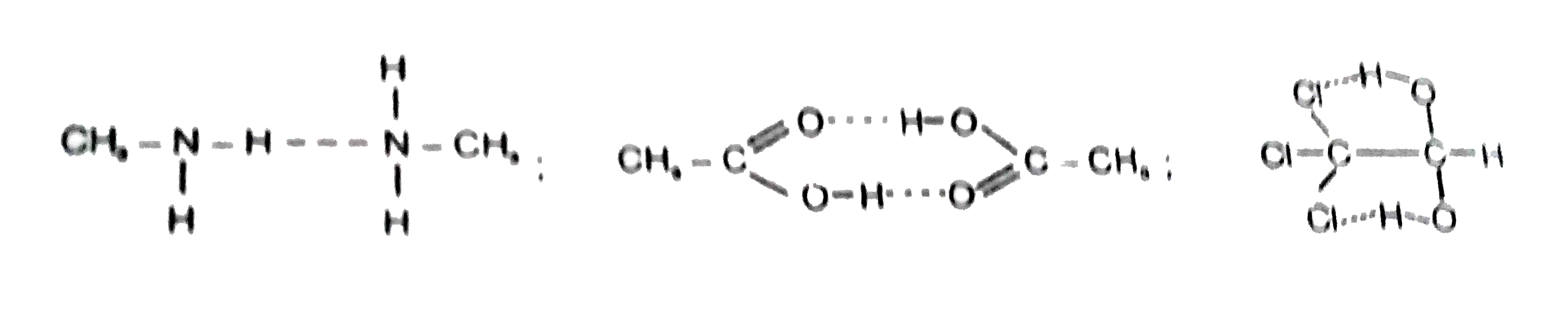
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-RANK BOOSTER-All Questions
- In which pairs is are the stronger bond found in the first species ?
Text Solution
|
- Which of the following statement is/are true ?
Text Solution
|
- Hydrogen bonding is present in which of the following species ?
Text Solution
|
- Assertion : F bond angle p is equal to the bond angle Q but not preci...
Text Solution
|
- Assertion Bond order in a molecule can assume any value positive integ...
Text Solution
|
- In the valence bond theory, hybridisation of orbitals is an integral p...
Text Solution
|
- The distribution of electrons among molecular orbitals is called the e...
Text Solution
|
- {:("Column-I","Column-II"),((A)XeF4,(p)sp^3d "see-saw geometry"),((B)S...
Text Solution
|
- The difference in the number of sigma and pi bond in trimer of SO3 i.e...
Text Solution
|
- How many hydrogen-bonded water molecule(s) are associated in CuSO(4).5...
Text Solution
|
- Sum of antibonding pi electrons (pi electrons ) in species O2, O2^(-) ...
Text Solution
|
- If the dipole moment of AB molecules is given by 2.4 D and A-B the bon...
Text Solution
|
- Match the complex ions listed in column-I with the characteristics lis...
Text Solution
|
- All the following complexes show decrease in their weights when placed...
Text Solution
|
- Which of the following statements is most likely to be incorrect ?
Text Solution
|
- s-1: [MnCl(6)]^(3),[FeF(6)]^(3-) and [CoF(6)]^(-3) are paramagnetic ha...
Text Solution
|
- Consider the following satements : S(1): Generally square planare c...
Text Solution
|
- Consider the following statements and arrange in the order of the true...
Text Solution
|
- Which of the following complexes is(are) paramagnetic ?
Text Solution
|
- For Mn^(+3) pairing energy is 28000 cm^(-1) , Delta0 for [Mn(CN)6]^(3-...
Text Solution
|