Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-RANK BOOSTER-All Questions
- How many of the following compounds has X-X bonds i.e., single coval...
Text Solution
|
- If Phosphorous acid, Tetrathionic acid and Pyrophosphoric acid have nu...
Text Solution
|
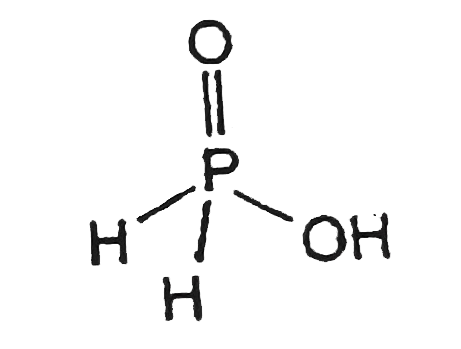
- The number of hydrogen atom(s) attached to phosphorus atom in hypophos...
Text Solution
|
- What is the sum of the oxidation state of phosphorus and number of P-O...
Text Solution
|
- How many mole of phosphine is obtained when one mole of calcuim phosph...
Text Solution
|
- In general the melting and boiling points of transition metals
Text Solution
|
- Which of the following is the most suitable description of transition ...
Text Solution
|
- Among the following the specials that is both paramagnetic and coloure...
Text Solution
|
- In which of the following oxoanions the oxidation state of central ato...
Text Solution
|
- Anhydrous ferric chloride is prepared by
Text Solution
|
- Ferric sulphate on heating gives:
Text Solution
|
- The ions from among the following which are colourless are : (i)Ti^(...
Text Solution
|
- Excess of KI reacts with CuSO(4) solution and Na(2)SO(3) solution is a...
Text Solution
|
- The yellow colour solution of Na(2)CrO(4) changes to orange red on pas...
Text Solution
|
- In general, the Transition elements exhibit their highest oxidation st...
Text Solution
|
- Which of the following products are formed when potassium bromide reac...
Text Solution
|
- Which of the following is a lanthanide ?
Text Solution
|
- Which of the following statement is not correct?
Text Solution
|
- The maximum oxidation state shown by V(Z=23),Cr(Z=24),Co(Z=27),Sc(Z=21...
Text Solution
|
- The most common oxidation states of cerium are
Text Solution
|