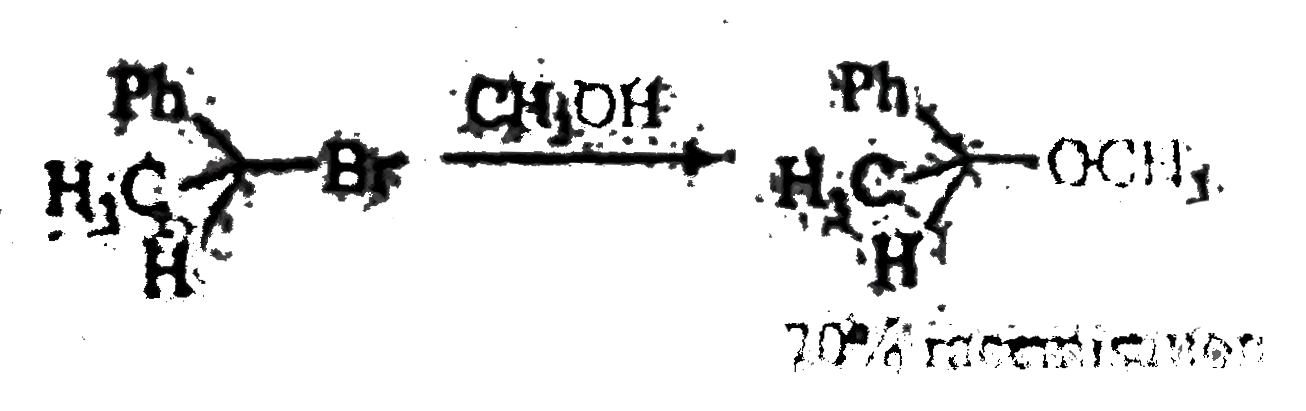A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
RESONANCE-RANK BOOSTER-All Questions
- Paul Walden, the brilliant son of a farmer succeeded in finding a conc...
Text Solution
|
- The reduction potential of hydrogen half cell will be negative if :
Text Solution
|
- A very thin copper plate is electro- plated with gold using gold chlor...
Text Solution
|
- We have taken a saturated solution of AgBr,K(sp) of AgBr is 12xx10^(-1...
Text Solution
|
- At what [Br^(-)]/sqrt(CO(3)^(2-)] does the following cell have its rea...
Text Solution
|
- Consider the cell Ag(s)|AgBr(s)Br^(c-)(aq)||AgCl(s),Cl^(c-)(aq)|Ag(s) ...
Text Solution
|
- For a saturated solution of AgCl at 25^(@)C,k=3.4xx10^(-6) ohm^(-1)cm^...
Text Solution
|
- With t taken in seconds and I taken in amp, the variation of I follows...
Text Solution
|
- You are given the followin cell at 298 K, Zn|(Zn^(+ +) .((aq.))),(0.01...
Text Solution
|
- A resistance of 50Omega is registered when two electrodes are suspende...
Text Solution
|
- the standard reduction potenital of a silver chloride electrode is 0.2...
Text Solution
|
- Calcualte the cell EMP in mV for Pt|H(2)(1 atm) | HCl(0.01 M)|| AgCl...
Text Solution
|
- Calculate the value of Lambda(m) ^prop for SrCl(2) in water at 25^(@)...
Text Solution
|
- Select the correct statement if- E(Mg^(2+)//Mg)^(@)=-2.4V, E(Sn^(4+)...
Text Solution
|
- For the cell (at 298K) Ag(s) | AgCl(s) | Cl^(-)(aq)|| AgNO(3)(aq) | ...
Text Solution
|
- Peroxodisulphate salts, (e.g., Na(2)S(2)O(8)) are strong oxidizing age...
Text Solution
|
- In which of the following cell(s): E("cell")=E("cell")^(@)?
Text Solution
|
- A current of 2.68A is passed for one hour through an aqeous solution o...
Text Solution
|
- Which is/are correct among the following? Given the half cell EMFs E...
Text Solution
|
- Mark out the correct statement(s) regarding electrolytic molar conduct...
Text Solution
|
- Statement-1:Zinc protect the iron better than tin even after it cracks...
Text Solution
|