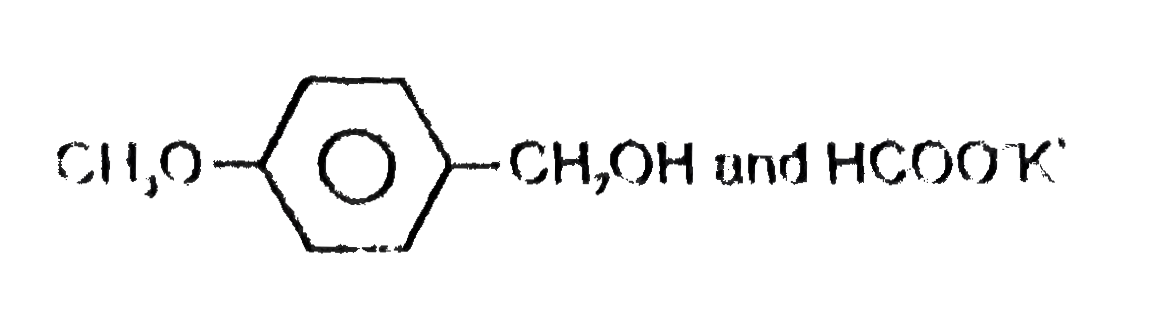
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
RESONANCE-RANK BOOSTER-All Questions
- Which one of the following compound will not show enolisation ?
Text Solution
|
- Statement-1:When chloral is heated with concentrated potassium hydroxi...
Text Solution
|
- Statement-1: Expected major product from the above reaction Statem...
Text Solution
|
- Statement-1:PhCOCOCOPh forms stable hydrate Statement-2:The compound...
Text Solution
|
- Reactivity order towards a nucleophile is
Text Solution
|
- When phenyl glyoxal is kept in a solution of aq. NaOH, it is converted...
Text Solution
|
- Complete the following reaction
Text Solution
|
- Complete the following reaction
Text Solution
|
Text Solution
|
- What statement is correct about the following reaction
Text Solution
|
- Identify C is the following sequence of reactions :
Text Solution
|
- Statement-1"C-O bond length is shorter in an ester as compared with an...
Text Solution
|
- Observe the following laboratory test for glucose pentacetate and ment...
Text Solution
|
- Nitrous acid (HNO(2)) converts amino acids into hydroxy acids with ret...
Text Solution
|
- Which of the following is condensation polymer ?
Text Solution
|
- Basic solution of fructose contains :
Text Solution
|
- Which one of the following is non-reducing sugar ?
Text Solution
|
- Glucose and mannose are :
Text Solution
|
- How many moles of acetic anhydride (Ac(2)O) is needed to react comple...
Text Solution
|
- Glucose on reduction with Na//Hg and water gives:
Text Solution
|
 Expected major product from the above reaction
Expected major product from the above reaction