Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS-PBQs
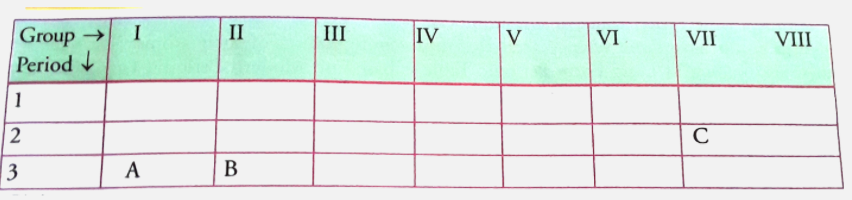
- Position of three elements A, B and C in the periodic table is shown b...
Text Solution
|
- A student dipped a strip of pH paper in distilled water taken in a tub...
Text Solution
|
- Five solutions A,B,C,D and E when tested with universal indicator show...
Text Solution
|
- Name the acid and base from which the following salts have been formed...
Text Solution
|
- When water is added gradually to a white solid X, a hissing sound is h...
Text Solution
|
- Two drinks P and Q gave acidic and alkaline reactions, respectively on...
Text Solution
|
- Take five test tubes and label them as A, B, C, D and E. Add 5 mL of f...
Text Solution
|
- An alkali metal A gives a compound B (molecular mass = 40) on reacting...
Text Solution
|
- You want to study a decomposition reaction by taking ferrous sulphate ...
Text Solution
|
- On keeping iron nails in a blue coloured copper sulphate solution, it ...
Text Solution
|
- While studying the double displacement reaction, the solutions of bari...
Text Solution
|
- You want to perform an experiment to study a double displacement react...
Text Solution
|
- You are given two colourless solutions present in two test tubes. One ...
Text Solution
|
- You are provided with two samples of hard water, one containing tempor...
Text Solution
|
- How will you distinguish between ethane and ethene with the help of a ...
Text Solution
|
- An unknown organic liquid does not turn blue litmus red and gives no e...
Text Solution
|
- How will you distinguish between hydrochloric acid and ethanoic acid w...
Text Solution
|
- Give a simple test to distinguish soaps from detergents.
Text Solution
|
- Write the name of apparatus/chemicals required to study the following ...
Text Solution
|
- A student added sodium hydrogen carbonate solution in ethanoic acid ta...
Text Solution
|
- A student is studying the properties of acetic acid in his school labo...
Text Solution
|