Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
DINESH PUBLICATION|Exercise Long Answer Questions|26 VideosPERIODIC CLASSIFICATION OF ELEMENTS
DINESH PUBLICATION|Exercise HIGHER ORDER THINKING SKILL BASED QUESTIONS|4 VideosPERIODIC CLASSIFICATION OF ELEMENTS
DINESH PUBLICATION|Exercise Very Short Answer Questions|32 VideosMETALS AND NON-METALS
DINESH PUBLICATION|Exercise MCQ|64 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS-Short Ansewer Questions
- Na, Mg and Al are the elements having one, two and three valence elect...
Text Solution
|
- Arrange the following elements in the descending order of atomic size ...
Text Solution
|
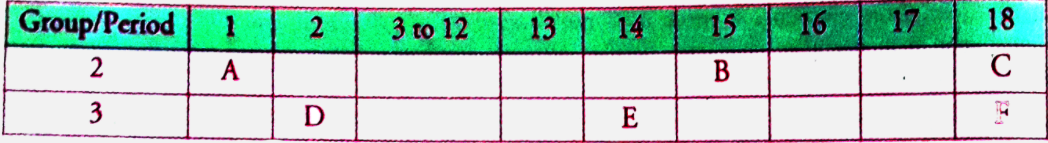
- The following table shows the positions of six elements A, B, C, D, E ...
Text Solution
|
- Given below are some elements of the modern periodic table : ""(4)Be...
Text Solution
|
- The elements Li, Na and K, each having one valence electron, are in pe...
Text Solution
|
- An element X (atomic number 17) combines with an element. Y (atomic nu...
Text Solution
|
- Two elements X and Y have atomic number 12 and 17 respectively. (i) ...
Text Solution
|
- Calcium forms the following salts : Calcium oxide -CaO, Calcium hydr...
Text Solution
|
- The atomic number of K and Ca is 19 and 20 respectively and they belon...
Text Solution
|
- Lithium, sodium and potassium are placed in the same group on the basi...
Text Solution
|
- (a) Name metals among first five elements of the Modern Periodic Table...
Text Solution
|
- As we move across a period in the periodic table, what is the gradatio...
Text Solution
|
- How does the tendency of the elements to lose electrons change in the ...
Text Solution
|
- An element 'X' belongs to 3^(rd) period and group 16 of the Modern, Pe...
Text Solution
|
- Three elements 'X', 'Y' and 'Z' have atomic numbers 7, 8 and 9 respect...
Text Solution
|
- An element 'X' belongs to 3^(rd) period and group 13 of the Modern Per...
Text Solution
|
- Compare and contrast the arrangement of elements in Mendeléev’s Period...
Text Solution
|
- Write the names given to the vertical columns and horizontal rows in t...
Text Solution
|
- Out of the elements H(1), Be(4), Na (11), Mg(12) : (a) Write the pai...
Text Solution
|
- The atomic number of an element is 19. (a) Write the electronic conf...
Text Solution
|