Text Solution
Verified by Experts
Topper's Solved these Questions
PERIODIC CLASSIFICATION OF ELEMENTS
DINESH PUBLICATION|Exercise Test Your Knowledge (Very Short Answer Questions)|15 VideosPERIODIC CLASSIFICATION OF ELEMENTS
DINESH PUBLICATION|Exercise Test Your Knowledge (Short Answer Questions)|14 VideosPERIODIC CLASSIFICATION OF ELEMENTS
DINESH PUBLICATION|Exercise Long Answer Questions|26 VideosMETALS AND NON-METALS
DINESH PUBLICATION|Exercise MCQ|64 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-PERIODIC CLASSIFICATION OF ELEMENTS-HIGHER ORDER THINKING SKILL BASED QUESTIONS
- Atoms of eight elements A, B, C, D, E, F, G and H have the same number...
Text Solution
|
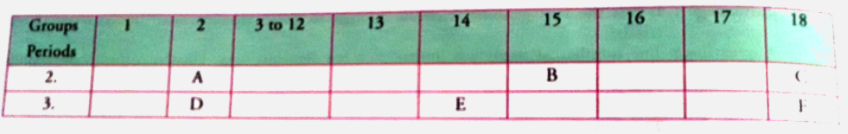
- The following table shows the position of six elements A, B, C, D, E a...
Text Solution
|
- Two elements X and Y belong to group 1 and 2 respectively in the same ...
Text Solution
|
- Atoms of seven elements A, B, C, D, E, F and G have a different number...
Text Solution
|