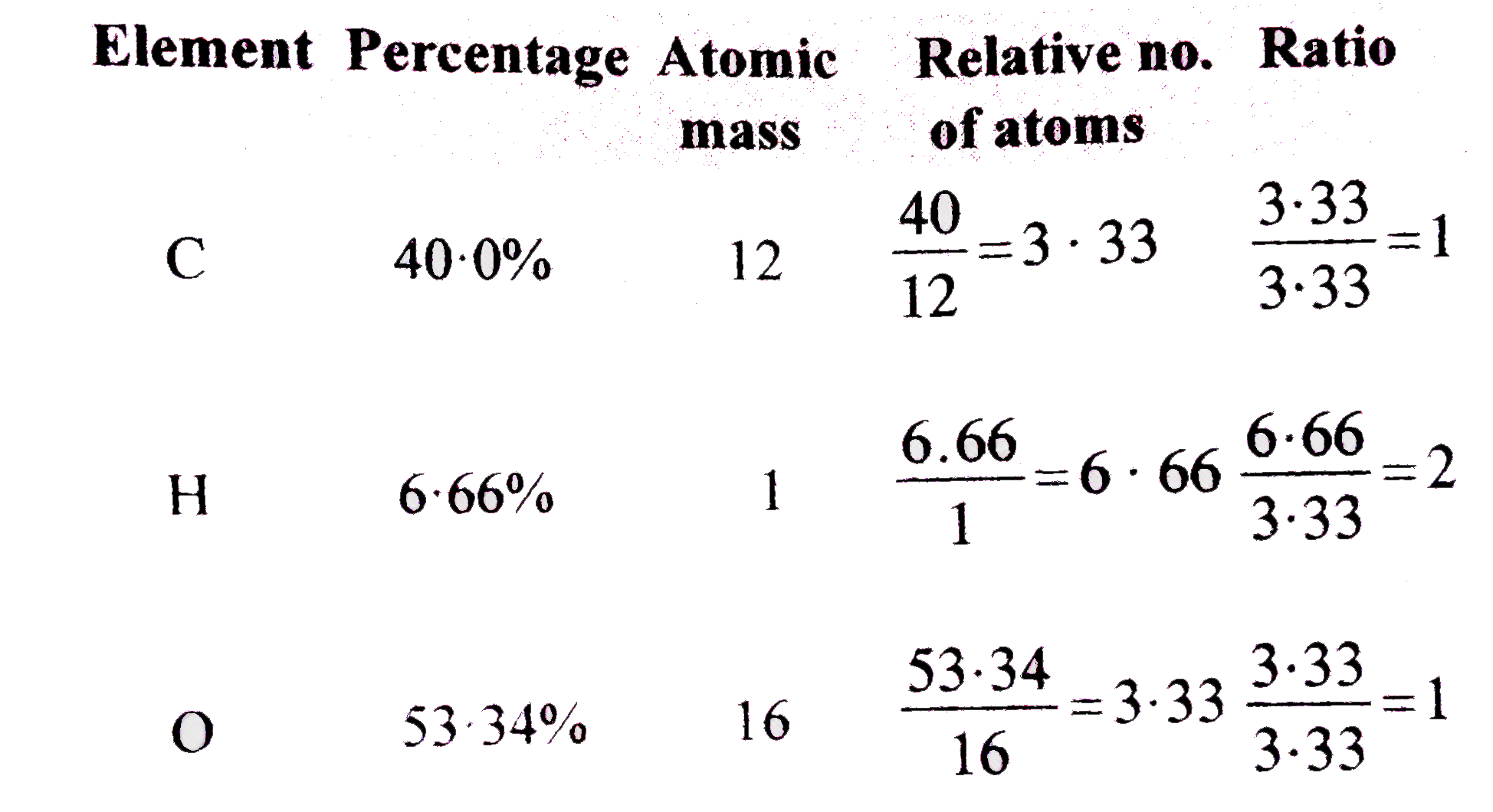A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND
DINESH PUBLICATION|Exercise Assorted .Q|21 VideosPURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND
DINESH PUBLICATION|Exercise Revision Q.F.Competitive exams|93 VideosPRINCIPLES RELATED TO PRACTICAL CHEMISTRY
DINESH PUBLICATION|Exercise Brain teasers-42|1 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Ultimate Preparation|9 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-PURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND -Ultimate Preparatory Package
- 60 g of oraganic compound on analysis gave following result C=24 g , H...
Text Solution
|
- The molecular mass of a compound containing only one nitrogen atom can...
Text Solution
|
- A gaseous hydrocarbon with empirical formula CH(2) has a density of 12...
Text Solution
|
- The haemoglobin contains 0:33% iron by weight. Its molecular mass is 6...
Text Solution
|
- The density of a gaseous organic compound is 1.3xx10^(-3) g mL^(-1) at...
Text Solution
|
- 9 " mL of " a mixture of methane and ethylene was exploded with 30 mL ...
Text Solution
|
- A compound which does not give a positive test in Lassaigne's test for...
Text Solution
|
- An organic compound contains, C, H and S. The minimum molecular weight...
Text Solution
|
- If 0.24 g of a volatile liquid upon vaporization gives 45 ml of vapors...
Text Solution
|
- A saturated hydrocarbon contains 82.66% carbon. Its molecular formula ...
Text Solution
|
- A purified pepsin was subjected to amino acid analysis. The amino acid...
Text Solution
|
- Thiophene can be removed from commercial benzene by
Text Solution
|
- An organic compound is extracted from its aqueous solution with 100 mL...
Text Solution
|
- Positive Beilstein test for halogen shows that
Text Solution
|
- A disbasic acid containing C, H, and O was found to contain C = 26.7% ...
Text Solution
|
- An organic compound has C and H percentage in the ratio 6:1 and C and ...
Text Solution
|