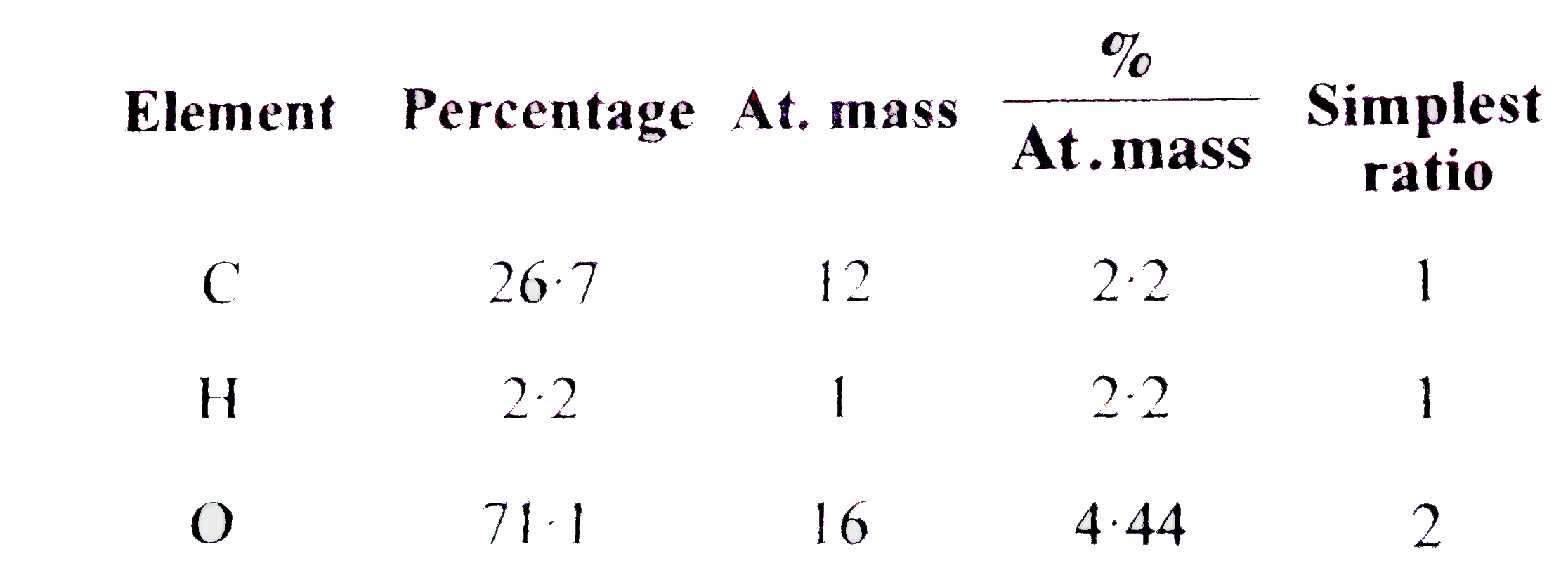A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND
DINESH PUBLICATION|Exercise SELECTED STRAIGHT MCQ|15 VideosPURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND
DINESH PUBLICATION|Exercise LINKED COMPREHENSION MCQ.|6 VideosPURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND
DINESH PUBLICATION|Exercise Assorted .Q|21 VideosPRINCIPLES RELATED TO PRACTICAL CHEMISTRY
DINESH PUBLICATION|Exercise Brain teasers-42|1 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Ultimate Preparation|9 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-PURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND -Revision Q.F.Competitive exams
- Absolute alcohol is prepared by
Text Solution
|
- Duma's method involves the determination of content of nitrogen in the...
Text Solution
|
- A dibasic acid containing C,H and O was found to contain C=26.7% and H...
Text Solution
|
- Leibing method is used for the estimation of
Text Solution
|
- In Lassaigne's test for nitrogen,the blue colour is due to the formati...
Text Solution
|
- Nitrogen in an organic compound can be estimated by
Text Solution
|
- Distillation is used to separate liquids which differ in their boiling...
Text Solution
|
- Which of the following organic compounds contains about 52 % carbon ?
Text Solution
|
- The purity of an organic compound is determined by
Text Solution
|
- During Lassaigne's test N and S present in an organic compound changes...
Text Solution
|
- Which of the follom'ng technique is most suitable for purification of ...
Text Solution
|
- The best method to separate the mixture of ortho and para nitrophenol ...
Text Solution
|
- Chloroform and benzene form a pair of miscible liquids. These can be s...
Text Solution
|
- Which one of the following is not used for the purification of solid i...
Text Solution
|
- The compound that does not give a blue colour is Lassaigne's test is
Text Solution
|
- Absolute alcohol is prepared by
Text Solution
|
- If 0.24 g of a volatile liquid upon vaporization gives 45 ml of vapour...
Text Solution
|
- An organic compound containing C,H and N gave the following analysis ...
Text Solution
|
- Emprical formula of a hydrocarbon containing 80 % corbon and 20 % hydr...
Text Solution
|
- If 0.2 g of an organic compound containing carbon,hydrogen and oxygen ...
Text Solution
|