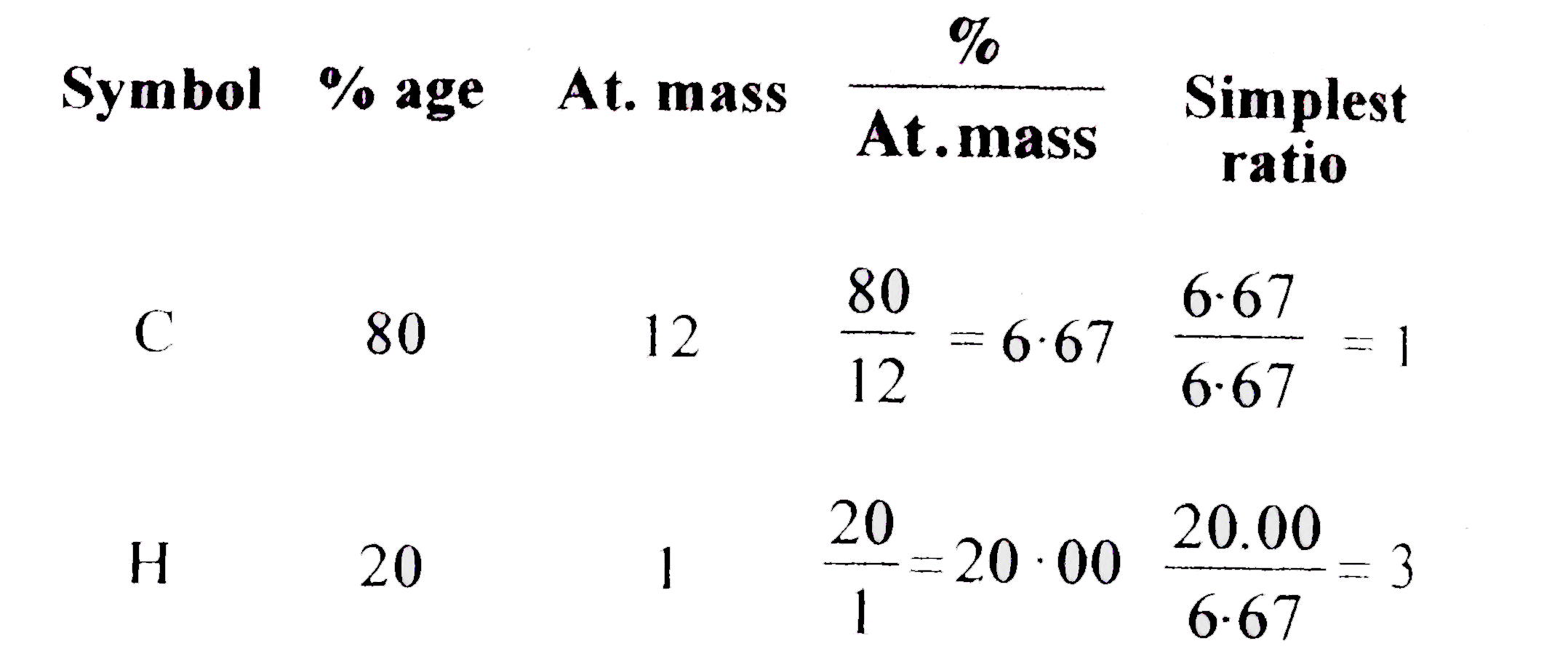A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND
DINESH PUBLICATION|Exercise SELECTED STRAIGHT MCQ|15 VideosPURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND
DINESH PUBLICATION|Exercise LINKED COMPREHENSION MCQ.|6 VideosPURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND
DINESH PUBLICATION|Exercise Assorted .Q|21 VideosPRINCIPLES RELATED TO PRACTICAL CHEMISTRY
DINESH PUBLICATION|Exercise Brain teasers-42|1 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Ultimate Preparation|9 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-PURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND -Revision Q.F.Competitive exams
- If 0.24 g of a volatile liquid upon vaporization gives 45 ml of vapour...
Text Solution
|
- An organic compound containing C,H and N gave the following analysis ...
Text Solution
|
- Emprical formula of a hydrocarbon containing 80 % corbon and 20 % hydr...
Text Solution
|
- If 0.2 g of an organic compound containing carbon,hydrogen and oxygen ...
Text Solution
|
- A mixture contains four solid organic compounds containing A, B, C an...
Text Solution
|
- In Kjedhal's method,, the nitrogen presence is estimed as
Text Solution
|
- An organic compound with C =40 % and H= 6.7% will have the empirical f...
Text Solution
|
- The equivalent wieght of an acid is equal to
Text Solution
|
- A compound with empirical formula O(2) has a vapour density of 30.Its ...
Text Solution
|
- The latest techique used for purification of organic compound is
Text Solution
|
- Empricial formula of a hydrocarbon containing 80 % carbon and 20% hy...
Text Solution
|
- Molecular mass of a volatile substance mat be obtained by
Text Solution
|
- An organic compound with C =40 % and H= 6.7% will have the empirical f...
Text Solution
|
- An organic compound with C =40 % and H= 13.33 % and N=46.67 % Its empi...
Text Solution
|
- Empirical formula of compound is CH(2)O.If its molecular weight is 180...
Text Solution
|
- The Beilstein test for organic compounds is used to detect
Text Solution
|
- Which of the following is the scientific method to test presence of wa...
Text Solution
|
- Which of the following has molecular weight of 92 ?
Text Solution
|
- Empirical formula of a compound is CH(2)O. If its vapour density is 90...
Text Solution
|
- Which of the following compounds does not show Lassaine's test for nit...
Text Solution
|