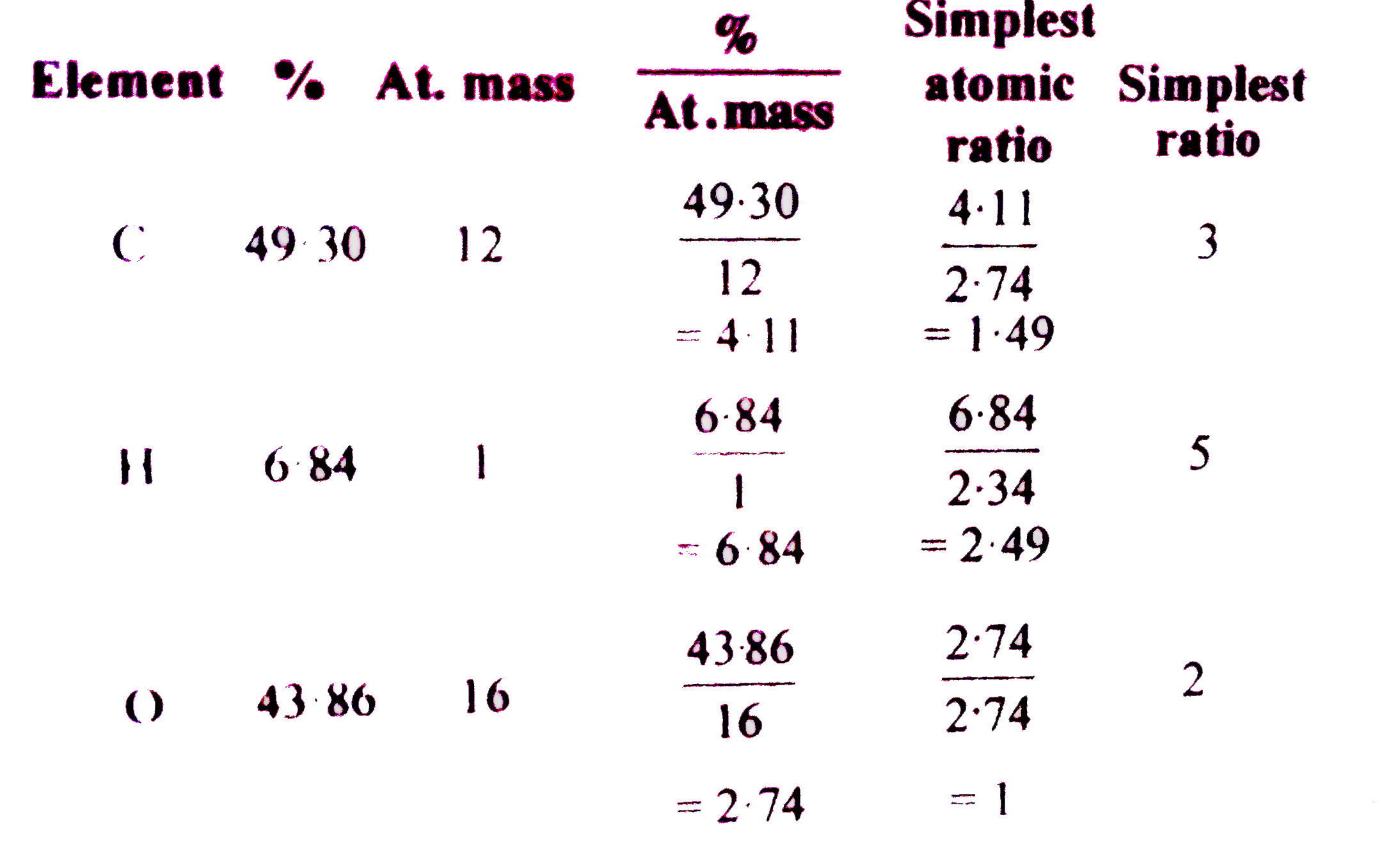A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
PURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND
DINESH PUBLICATION|Exercise SELECTED STRAIGHT MCQ|15 VideosPURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND
DINESH PUBLICATION|Exercise LINKED COMPREHENSION MCQ.|6 VideosPURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND
DINESH PUBLICATION|Exercise Assorted .Q|21 VideosPRINCIPLES RELATED TO PRACTICAL CHEMISTRY
DINESH PUBLICATION|Exercise Brain teasers-42|1 VideosREDOX REACTIONS
DINESH PUBLICATION|Exercise Ultimate Preparation|9 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-PURIFICATION & CHARACTERISATION OF ORGANIC COMPOUND -Revision Q.F.Competitive exams
- Which of the following compounds does not show Lassaine's test for nit...
Text Solution
|
- 0.1914 g of an organic acidi s dissolved in about 20 ml of water. 25 ...
Text Solution
|
- An organic compound contains 49.3 % carbon,6.84 % hydrogen and its vap...
Text Solution
|
- The empirical formula of an acid is CH(2)O(2) the probable molecular f...
Text Solution
|
- In paper chromatography,
Text Solution
|
- If 0.765 g of an acid gives 0.535 g of CO(2) and 0.138 g of H(2)O, the...
Text Solution
|
- Percentage of Se (at. mass 3 78.4) in peroxidase anhydrase enzyme is 0...
Text Solution
|
- In a hydrocarbon, mass ratio of hydrogen and carbon is 1 : 3, the empi...
Text Solution
|
- The empirical formula of a compound is CH2 . One mole of compound has ...
Text Solution
|
- An organic compound containing carbon hydrogen and oxygen contains 52 ...
Text Solution
|
- In a compound C, H, N atoms are present in 9:1:3.5 by weight. Molecula...
Text Solution
|
- Empirical formula of a compound is CH(2)O and its molecular mass is 9...
Text Solution
|
- An organic compound on analysis gave C=39.9 % ,H= 6.7 % and O =53.4 % ...
Text Solution
|
- Which of the following statements is wrong ?
Text Solution
|
- If we want to study the relative arrangement of atoms in a molecule, w...
Text Solution
|
- In Victor Meyers method, 0.2 g of an organic substance displaced 56 mL...
Text Solution
|
- 116 mg of a compound on vaporisation in a victor Meyer's apparatus dis...
Text Solution
|
- The compound formed in the positive test for neitrogen with Lassaigne ...
Text Solution
|
- The ammonia evolved from the treatment of 0.30 g of an organic compoun...
Text Solution
|
- Sodium nitoprusside when added to an alkaline solution of sulphide ion...
Text Solution
|