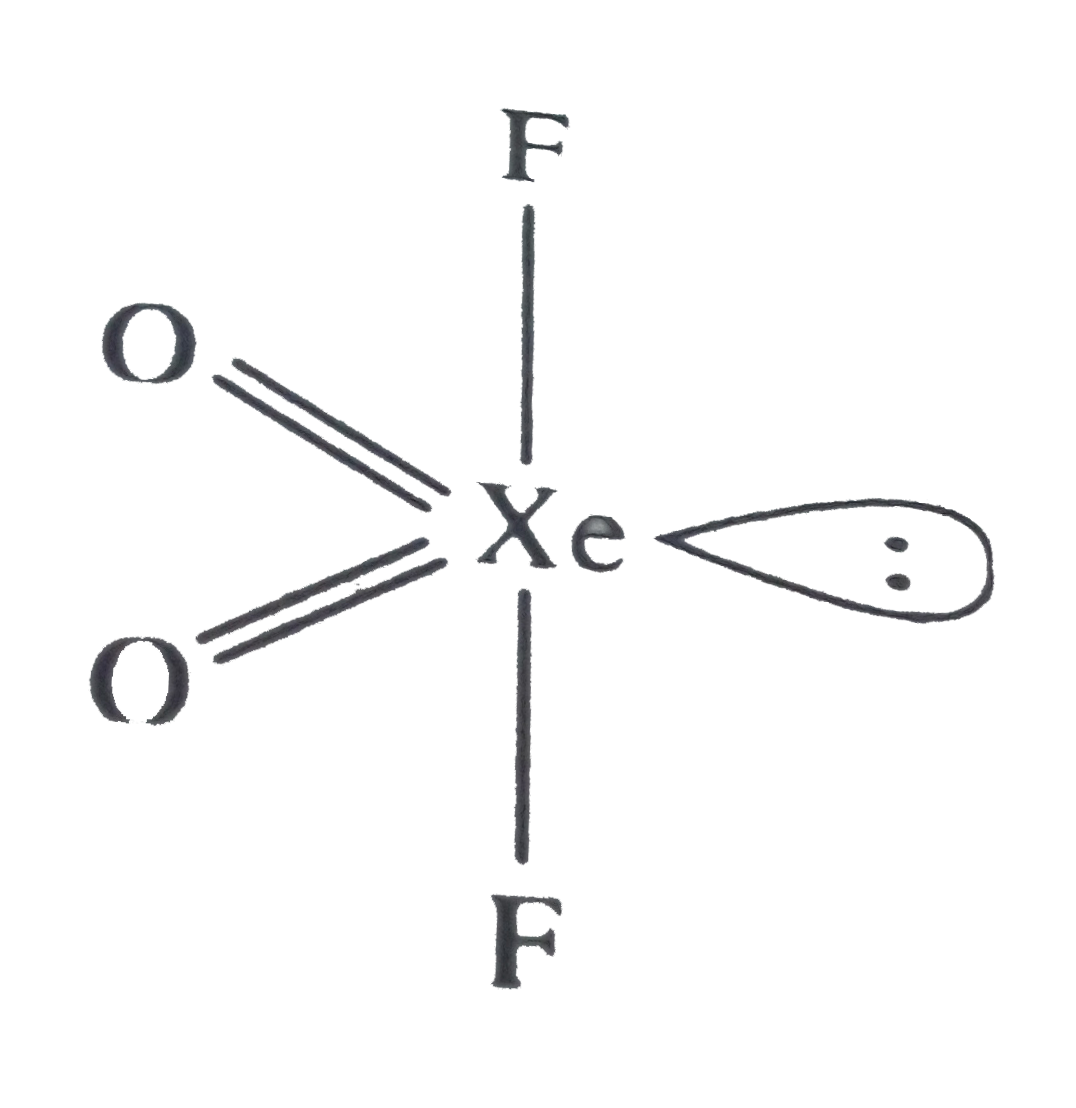
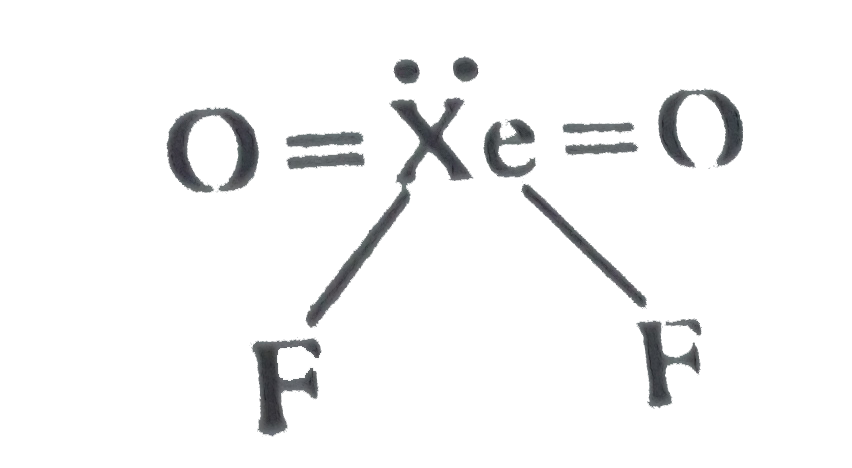A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE NOBLE GASES
DINESH PUBLICATION|Exercise Linked Comprehension Type MCQ|18 VideosTHE NOBLE GASES
DINESH PUBLICATION|Exercise Matrix Match Type|5 VideosTHE NOBLE GASES
DINESH PUBLICATION|Exercise Brain Teasers - 20|40 VideosTHE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Ultimate|14 VideosTHE OXYGEN FAMILY
DINESH PUBLICATION|Exercise PREPARATORY PACKAGE|14 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE NOBLE GASES-RQ
- Which of the following is not correct ?
Text Solution
|
- The molecular shapes of SF(4),CF(4) " and " XeF(4) are :
Text Solution
|
- Among the following molecules, (i)XeO(3)(ii)XeOF(4)(iii)XeF(6) those h...
Text Solution
|
- Which inert gas has abnormal behaviour in liquefaction
Text Solution
|
- The formation of O(2)^(+)[PtF(6)]^(-) is the basis for the formation o...
Text Solution
|
- Hydrolysis of XeF(4) and CaNCN gives respectively :
Text Solution
|
- Among the following , the pair in which the two species are not isostr...
Text Solution
|
- Which is the most easily liquifiable rare gas
Text Solution
|
- Which one of the following reaction of xenon compounds is not Feasible...
Text Solution
|
- Perxenate ion is
Text Solution
|
- Which of the following fluorides of Xe has zero dipole moment ?
Text Solution
|
- Which of the following contains maximum number of pairs around Xe atom...
Text Solution
|
- Which of the following noble gases is used in miner's cap lamp ?
Text Solution
|
- The structure of XeO(2)F(2) is
Text Solution
|
- Which of the following is used in flash tubes in photography ?
Text Solution
|
- Which of the followng noble gases has the highest positive electron ga...
Text Solution
|
- XeF(2) is iso-structural with
Text Solution
|
- Low chemical reactivity of the noble gases can be attributed to their
Text Solution
|
- Argon is used
Text Solution
|
- The molecular shapes of SF(4),CF(4) " and " XeF(4) are :
Text Solution
|