A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THE NOBLE GASES
DINESH PUBLICATION|Exercise Matrix Match Type|5 VideosTHE NOBLE GASES
DINESH PUBLICATION|Exercise Assertion Reason|10 VideosTHE NOBLE GASES
DINESH PUBLICATION|Exercise RQ|96 VideosTHE NITROGEN FAMILY
DINESH PUBLICATION|Exercise Ultimate|14 VideosTHE OXYGEN FAMILY
DINESH PUBLICATION|Exercise PREPARATORY PACKAGE|14 Videos
Similar Questions
Explore conceptually related problems
DINESH PUBLICATION-THE NOBLE GASES-Linked Comprehension Type MCQ
- There are some deposits of nitrated and phosphates in the earth's crus...
Text Solution
|
- There are some deposits of nitrates and phosphates. Nitrates are more ...
Text Solution
|
- There are some deposits of nitrates and phosphates in the earth's crus...
Text Solution
|
- The noble gases are chemically inert due to high ionization enthalpy, ...
Text Solution
|
- The noble gases are chemically inert due to high ionization enthalpy, ...
Text Solution
|
- The noble gases are chemically inert due to high ionization enthalpy, ...
Text Solution
|
- The noble gases are chemically inert due to high ionization enthalpy, ...
Text Solution
|
- The name 'silica' covers an entire group of minerals which have the ge...
Text Solution
|
- The name 'silica' covers an entire group of minerals which have the ge...
Text Solution
|
- The name 'silica' covers an entire group of minerals which have the ge...
Text Solution
|
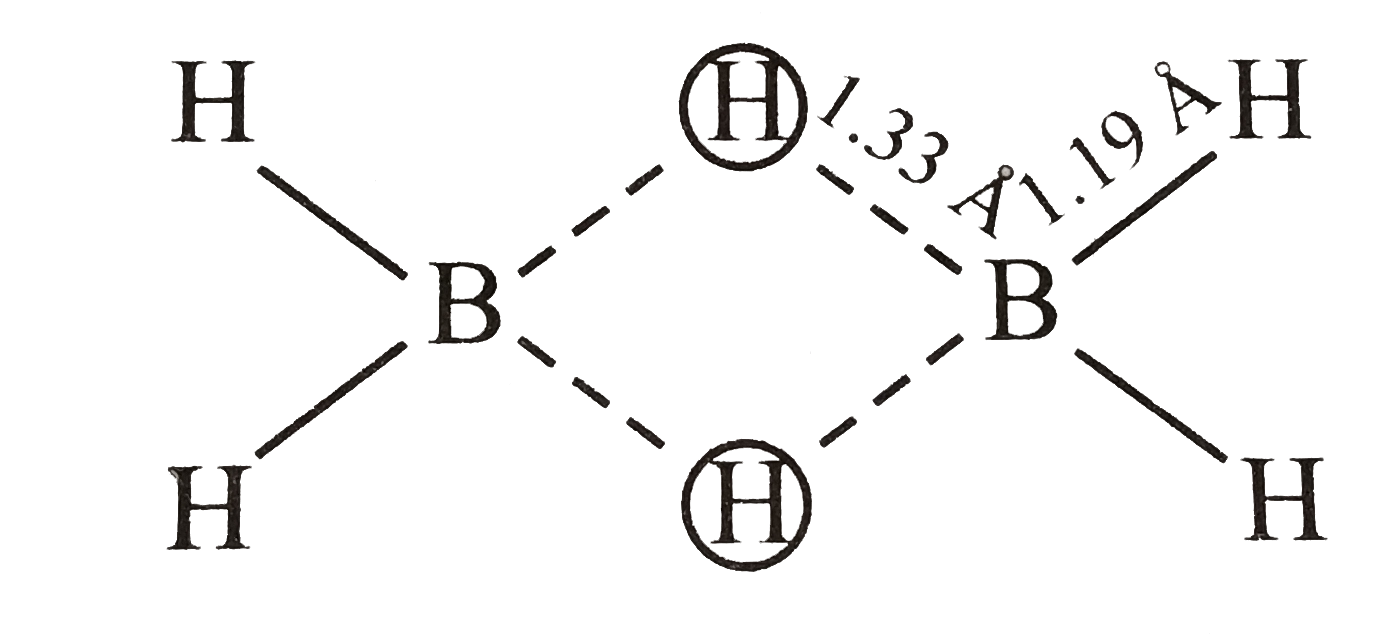
- The molecular shapes of diborane is shown below : . Consider the f...
Text Solution
|
- The molecular shapes of diborane is shown below : . Select correct...
Text Solution
|
- The noble gases have closed-shell electronic cordigaration and are mon...
Text Solution
|
- The noble gases have closed-shell electronic cordigaration and are mon...
Text Solution
|
- XeF(4) and XeF(6) are expected to be
Text Solution
|
- An oxy acid of phosphorus has the following properties. Complete neu...
Text Solution
|
- An oxy acid of phosphorus has the following properties. Complete neu...
Text Solution
|
- An oxy acid of phosphorus has the following properties. Complete neu...
Text Solution
|
 .
.