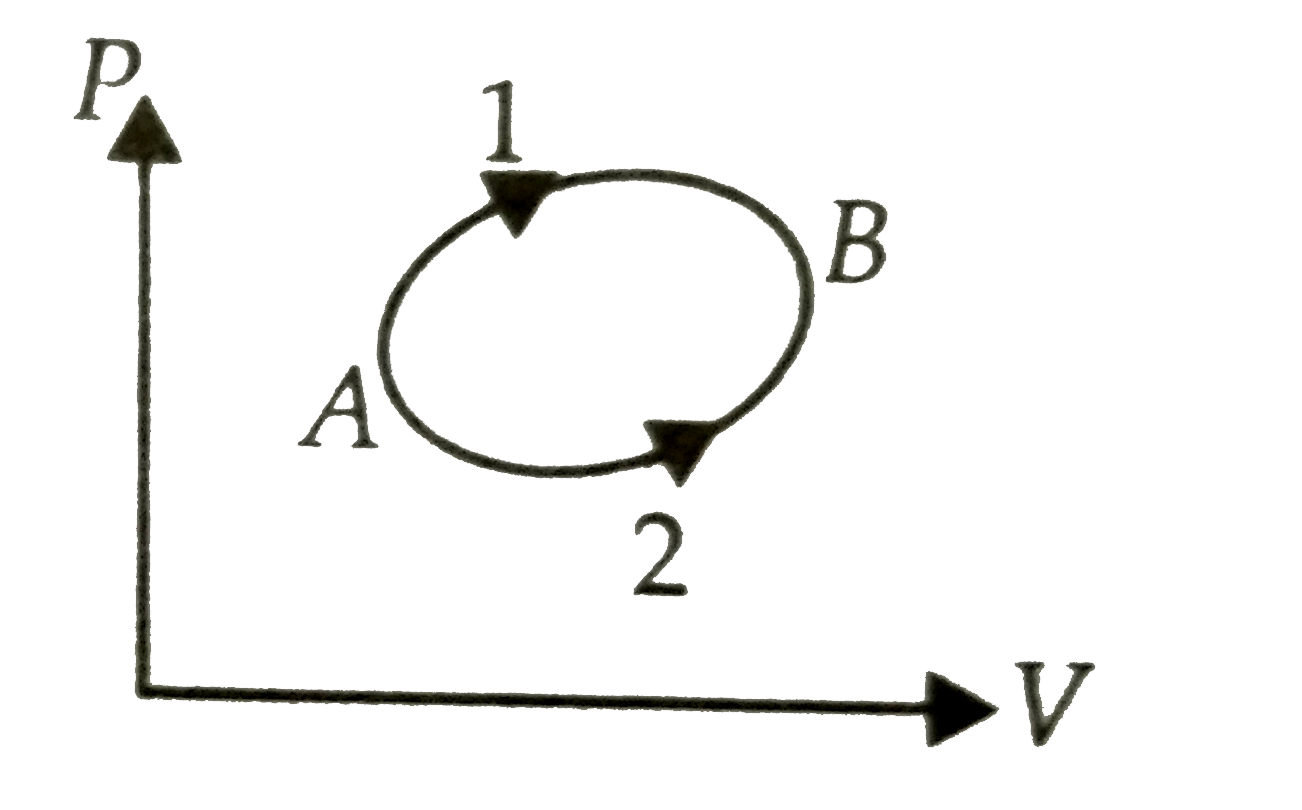
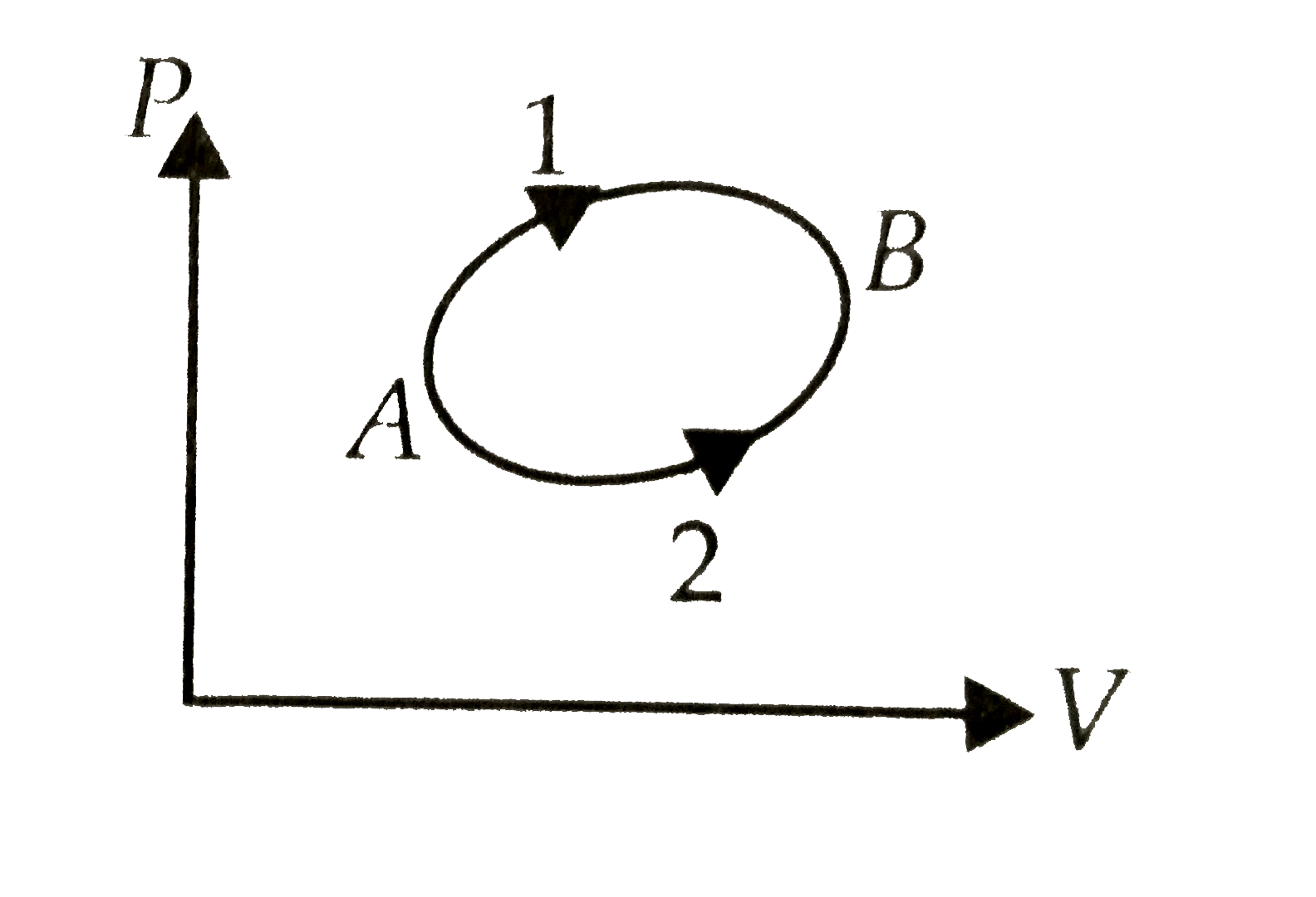
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
NCERT FINGERTIPS|Exercise Specific Heat Capacity|14 VideosTHERMODYNAMICS
NCERT FINGERTIPS|Exercise Thermodynamic State Variables|2 VideosTHERMODYNAMICS
NCERT FINGERTIPS|Exercise Heat, Internal Energy And Work|2 VideosTHERMAL PROPERTIES OF MATTER
NCERT FINGERTIPS|Exercise Assertion And Reason|10 VideosUNITS AND MEASUREMENTS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-THERMODYNAMICS-First Law Of Thermodynamics
- Which of the following is not a path function ?
Text Solution
|
- Air is expanded from 50 litres to 150 litres at 2 atomospheric pressur...
Text Solution
|
- An electric heater supplies heat to a system at a rate of 120 W. if sy...
Text Solution
|
- 1 kg of water is heated from 40^(@) C to 70^(@)C, If its volume remain...
Text Solution
|
- A system goes from A to B by two different paths in the P-V diagram a...
Text Solution
|