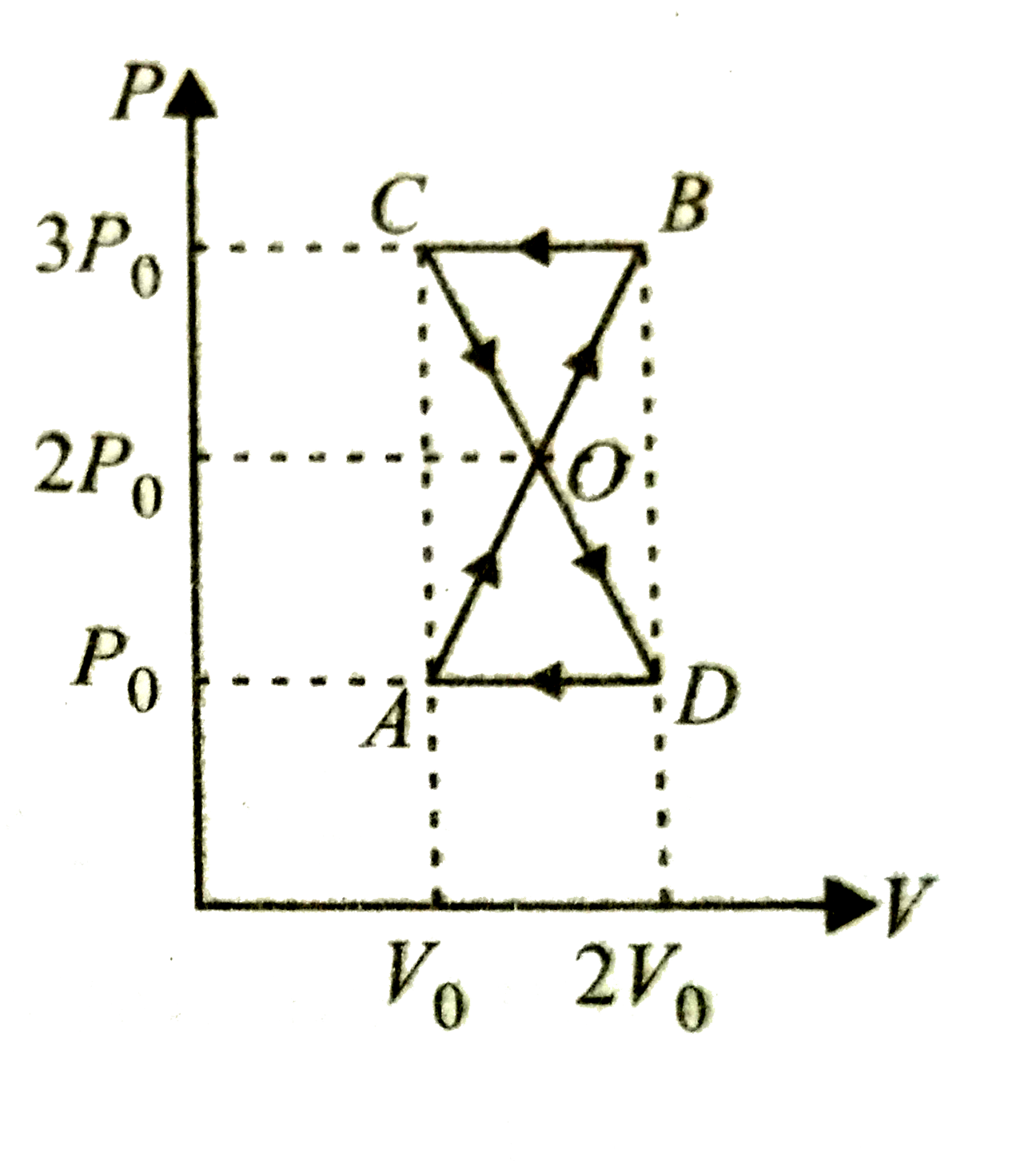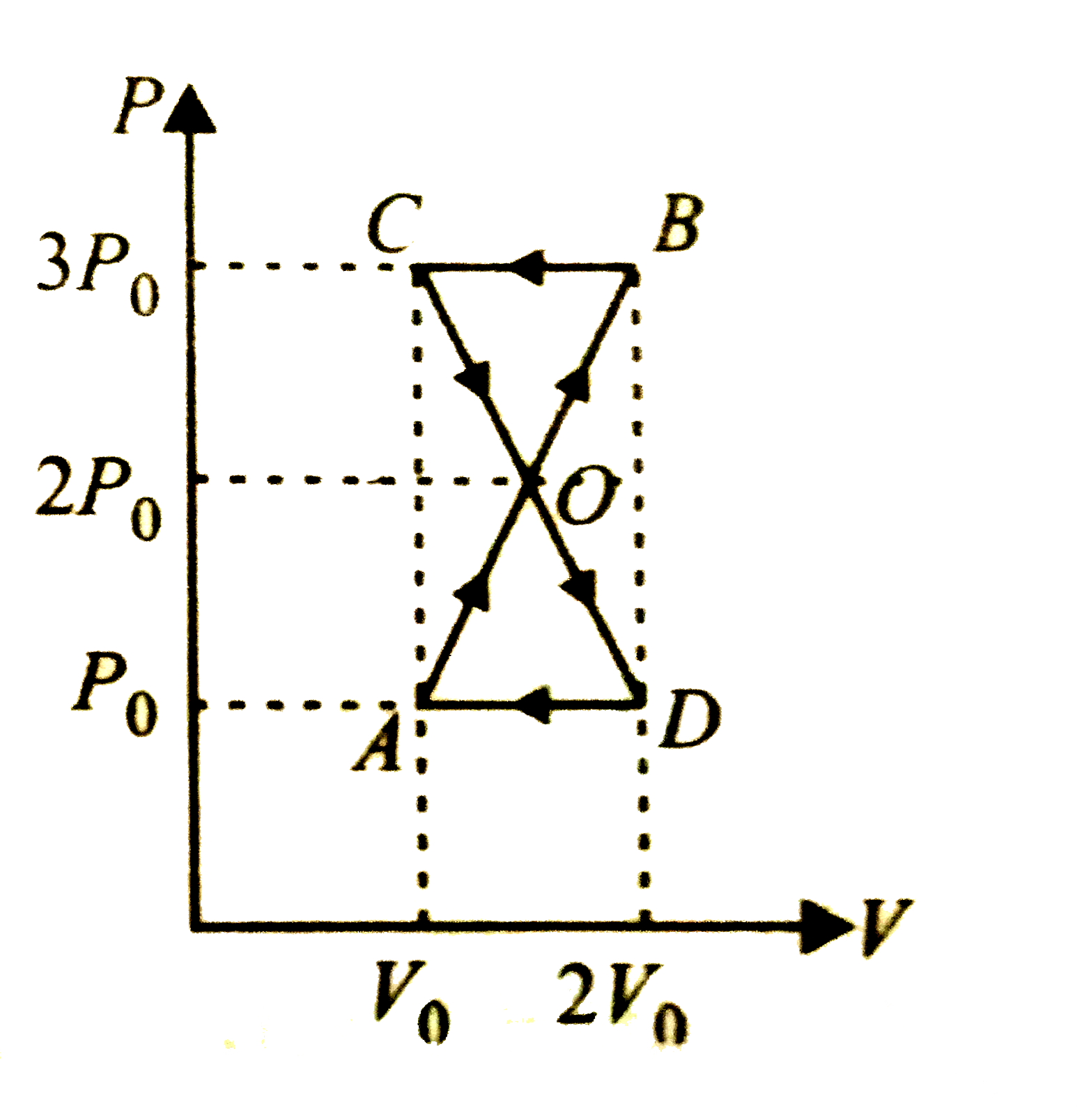A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
NCERT FINGERTIPS|Exercise Heat Engines|4 VideosTHERMODYNAMICS
NCERT FINGERTIPS|Exercise Refrigerators And Heat Pumps|10 VideosTHERMODYNAMICS
NCERT FINGERTIPS|Exercise Thermodynamic State Variables|2 VideosTHERMAL PROPERTIES OF MATTER
NCERT FINGERTIPS|Exercise Assertion And Reason|10 VideosUNITS AND MEASUREMENTS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-THERMODYNAMICS-Thermodynamic Process
- Two moles of an ideal monoatomic gas occupy a volume 2V at temperature...
Text Solution
|
- The pressure P(1) and density d(1) of a diatomic gas (gamma=(7)/(5)) c...
Text Solution
|
- The fall in temperature of helium gas initially at 20^(@) when it is...
Text Solution
|
- A cycle followed an engine (made of one mole of an ideal gas in a cyl...
Text Solution
|
- In the question number 61, the heat exchanged by the engine with the ...
Text Solution
|
- A one mole of an ideal gas expands adiabatically at a constant pressur...
Text Solution
|
- A gas is suddenly compressed to 1/4th of its original volume. Caculate...
Text Solution
|
- 1 mole of gas expands isothermally at 37^(@)C. The amount of heat is a...
Text Solution
|
- The temperature of n moles of an ideal gas is increased from T to 4T t...
Text Solution
|
- A gas expands with temperature according to the relation V=KT^(2/3).Wo...
Text Solution
|
- 50g of oxygen at NTP is compressed adiabatically to a pressure of 5 at...
Text Solution
|
- In a cyclic process, which of the following statement is correct?
Text Solution
|
- Which one of the following is not possible in a cyclic process?
Text Solution
|
- A thermodynamic process is carried out from an original state D to an ...
Text Solution
|
- One mole of an ideal gas undergoes a cyclic process ABCDA as shown in ...
Text Solution
|
- The cycle is shown in figure is made of one mole of perfect gas in a c...
Text Solution
|
- Two moles of Helium gas undergo a reversible cyclic process as shown i...
Text Solution
|
- The cyclic process for 1 mole of an ideal gas is shown in the V-T diag...
Text Solution
|
- The heat absorbed by the system in going through the cyclic process as...
Text Solution
|
- A thermodynamical system undergoes cyclic process ABCDA as shown in fi...
Text Solution
|