A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
NCERT FINGERTIPS|Exercise Carnot Engine|9 VideosTHERMODYNAMICS
NCERT FINGERTIPS|Exercise Higher Order Thinking Skills|8 VideosTHERMODYNAMICS
NCERT FINGERTIPS|Exercise Heat Engines|4 VideosTHERMAL PROPERTIES OF MATTER
NCERT FINGERTIPS|Exercise Assertion And Reason|10 VideosUNITS AND MEASUREMENTS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-THERMODYNAMICS-Refrigerators And Heat Pumps
- When the door of a refrigerator is kept open then the room temperature...
Text Solution
|
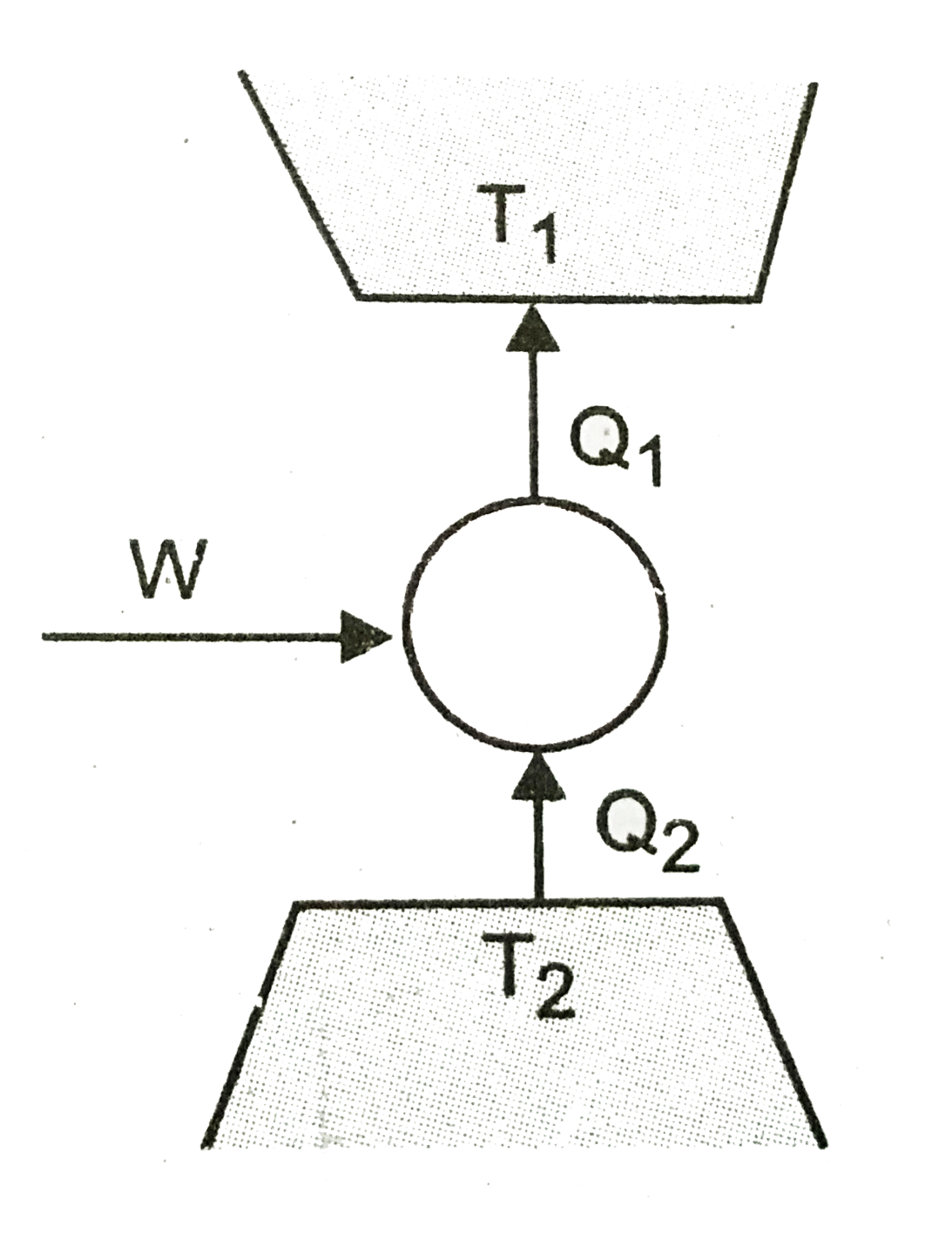
- Consider a heat engine as shown in (figure). Q(1) and Q(2) are heat ad...
Text Solution
|
- A refrigerator is to maintain eatables kept inside at 7^(@)C. The coef...
Text Solution
|
- The coefficient of performance of refrigerator, whose efficiencty is ...
Text Solution
|
- If the coefficient of performance of a refrigerator is 5 and operates ...
Text Solution
|
- The temperature inside a refrigerator is t(2)^(@)C . The amount of hea...
Text Solution
|
- The reezer in a refrigeratror is located at the top section so that
Text Solution
|
- A refrigerator with COP= 1//3 release 200 J at heat to a reservoir. Th...
Text Solution
|
- A process is said to be reversible if
Text Solution
|
- Which of the processes described below are irreversible ?
Text Solution
|