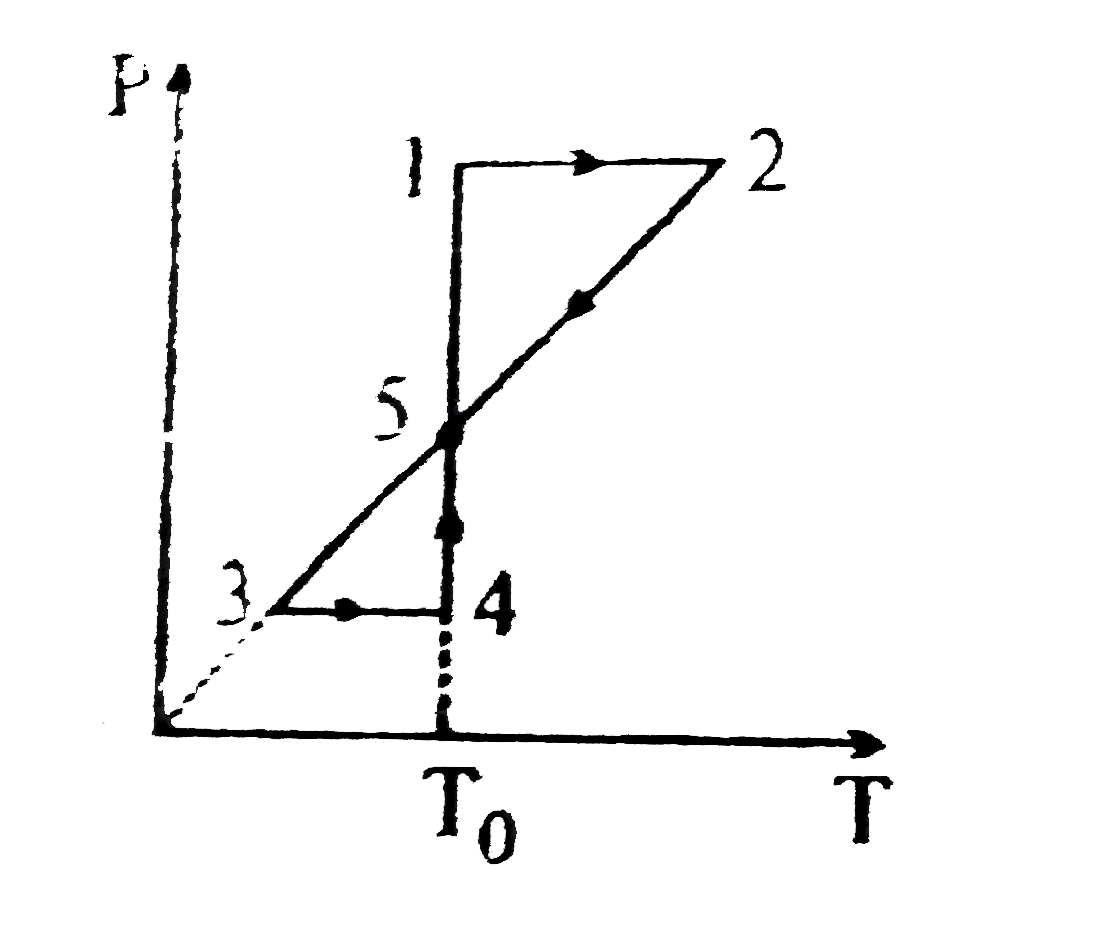
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS
NCERT FINGERTIPS|Exercise MCQs|6 VideosTHERMODYNAMICS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 VideosTHERMODYNAMICS
NCERT FINGERTIPS|Exercise Carnot Engine|9 VideosTHERMAL PROPERTIES OF MATTER
NCERT FINGERTIPS|Exercise Assertion And Reason|10 VideosUNITS AND MEASUREMENTS
NCERT FINGERTIPS|Exercise Assertion And Reason|15 Videos
Similar Questions
Explore conceptually related problems
NCERT FINGERTIPS-THERMODYNAMICS-Higher Order Thinking Skills
- A thermodynamic process of one mole ideal monoatomic gas is shown in f...
Text Solution
|
- Some gas (C(p)//C(V)=gamma=1.25) follows the cycle ABCDA as shown in t...
Text Solution
|
- For an ideal gas the equation of a process for which the heat capacity...
Text Solution
|
- A monatomic ideal gas is following the cyclic proces ABCA. Then choose...
Text Solution
|
- Consider PT graph of cyclic process shown in the figure. Maximum press...
Text Solution
|
- In the question number 5, if the maiximum pressure is P then what is t...
Text Solution
|
- One mole of a monatomic ideal gas is taken through the cycle shown in ...
Text Solution
|
- A gaseous mixture enclosed in a vessel of volume V consists of one gra...
Text Solution
|