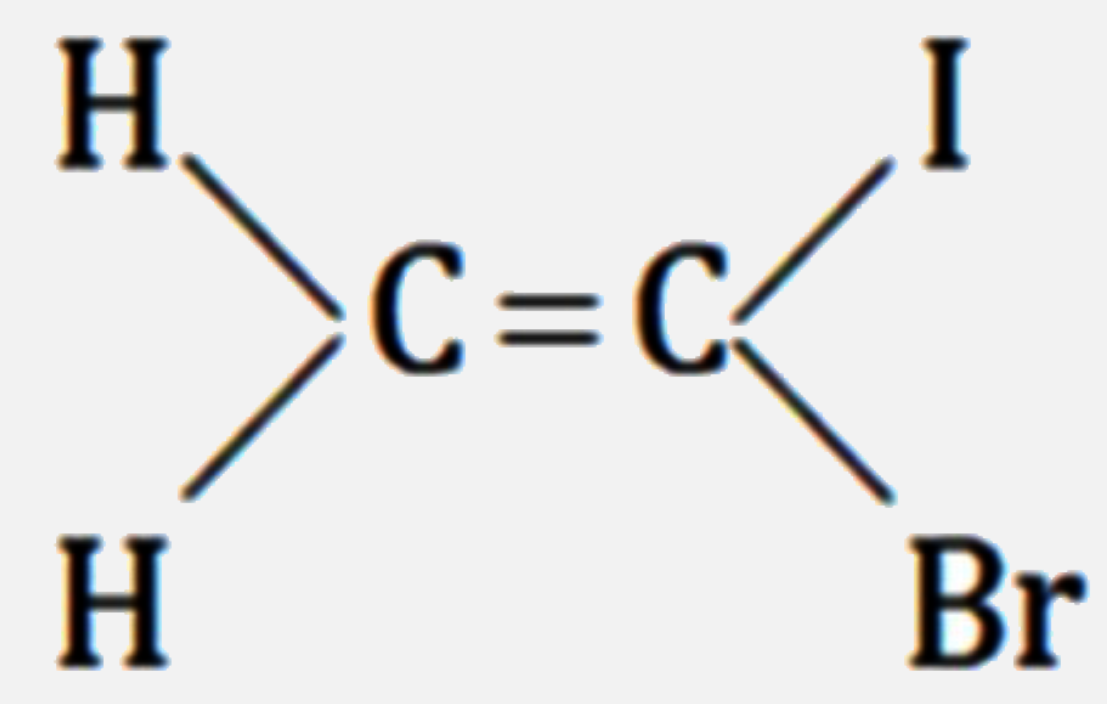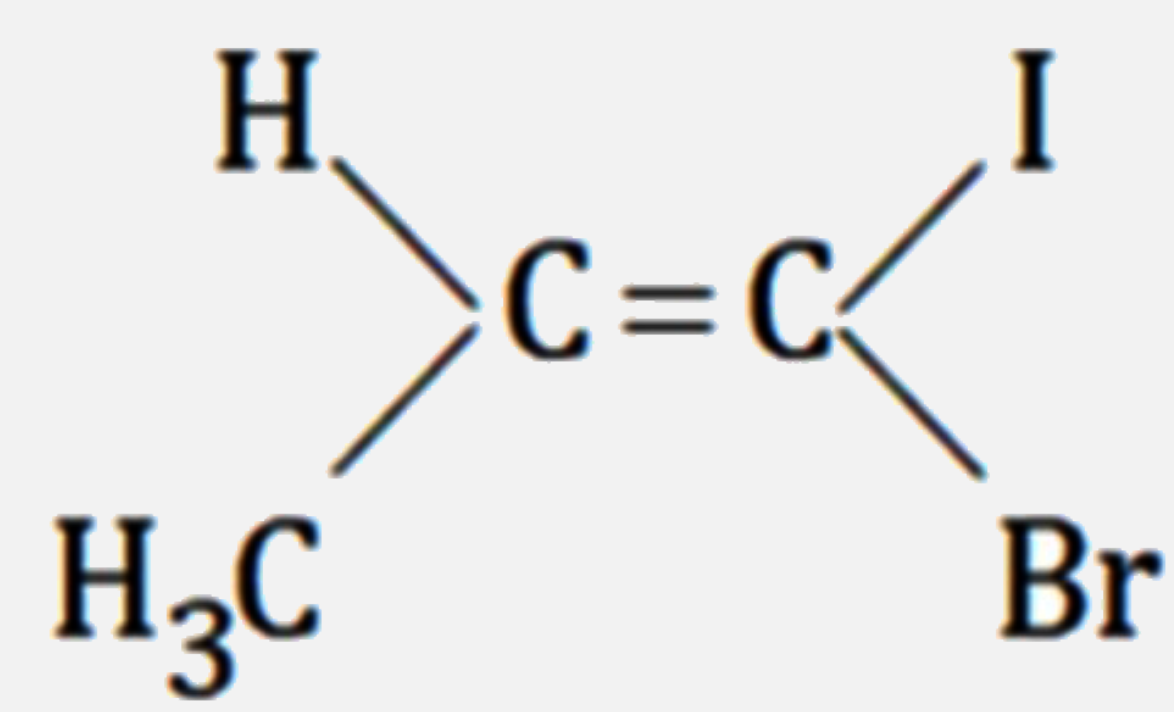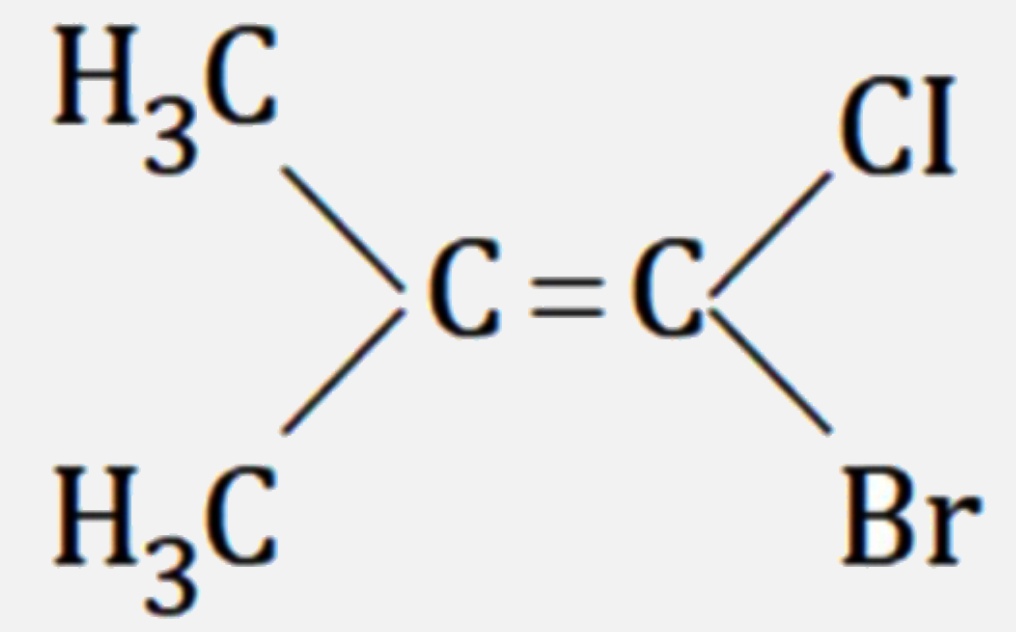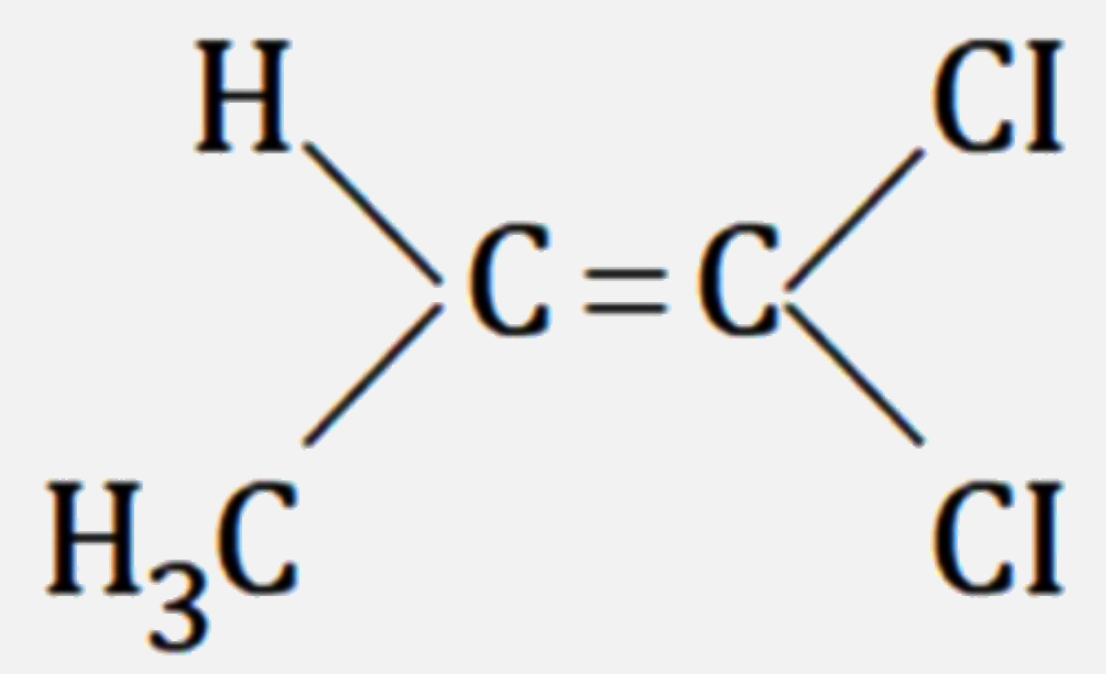A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 74-CHEMISTRY
- Which of the following statement is incorrect ?
Text Solution
|
- Alkali metals impart colour to Bunsen flame due to
Text Solution
|
- Geometrical isomerism is show by
Text Solution
|
- At the same temperature and pressure ,which of the following gases wil...
Text Solution
|
- Which of the following is the most stable carbanion?
Text Solution
|
- Which member of group 13 does not exhibit the group valency in its com...
Text Solution
|
- Body centred cubic and face centred cubic unit cells have n1 and n2 e...
Text Solution
|
- Which of the following carbides reacts with H2O to form propyne ?
Text Solution
|
- The reaction of t - butyl chloride and sodium ethoxide gives mainly
Text Solution
|
- Which of the following is not an ionic halide ?
Text Solution
|
- The equilibrium constant for the reaction N(2)(g)+O(2)(g) hArr 2NO(g) ...
Text Solution
|
- Which of the following would cause the percent ionization of week acid...
Text Solution
|
- Sodium reacts with alcohol as given below 2Na+ 2ROH rarrH2+2[Na^(+) ba...
Text Solution
|
- The possible numerof structural and stereo isomers for the complex [MB...
Text Solution
|
- The hydrocarbon (molecular mass = 70) after reduction and chlorination...
Text Solution
|
- The dissociation energies of H2and O2 are 104 and 118 " kcal mol"^(-1...
Text Solution
|
- Which of the following plots represents correctly variation of equival...
Text Solution
|
- Which bond angle theta would result in the maximum dipole moment for t...
Text Solution
|
- Which of the following sets of quantum numbers represent an impossible...
Text Solution
|
- On adding KMnO(4) to cold conc. H(2)SO(4), it gives
Text Solution
|