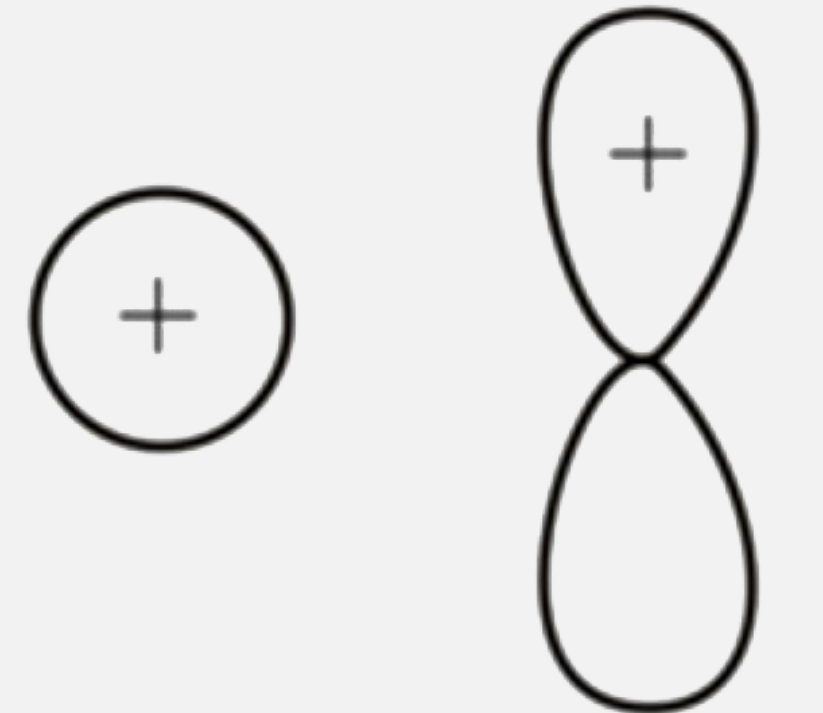
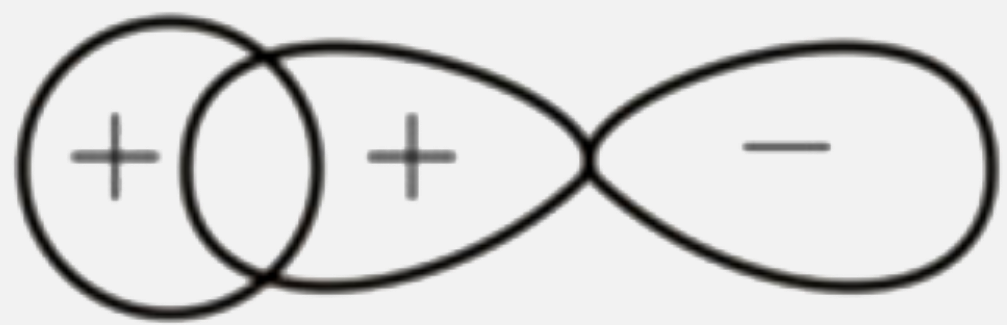
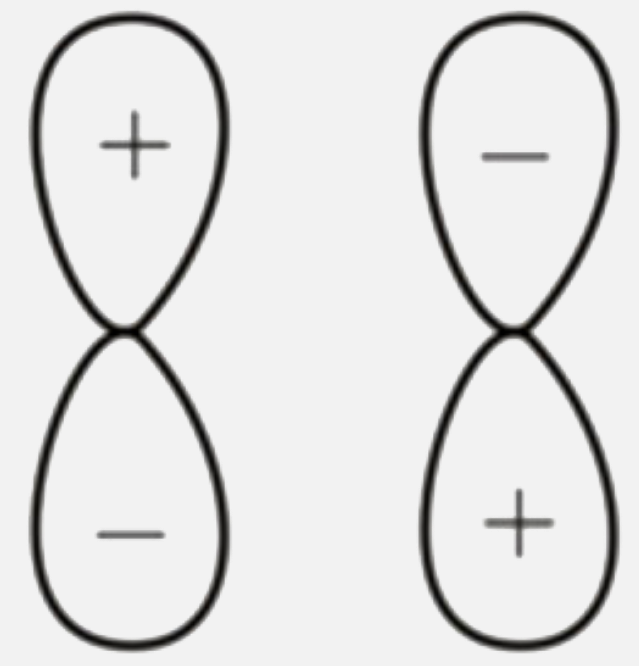
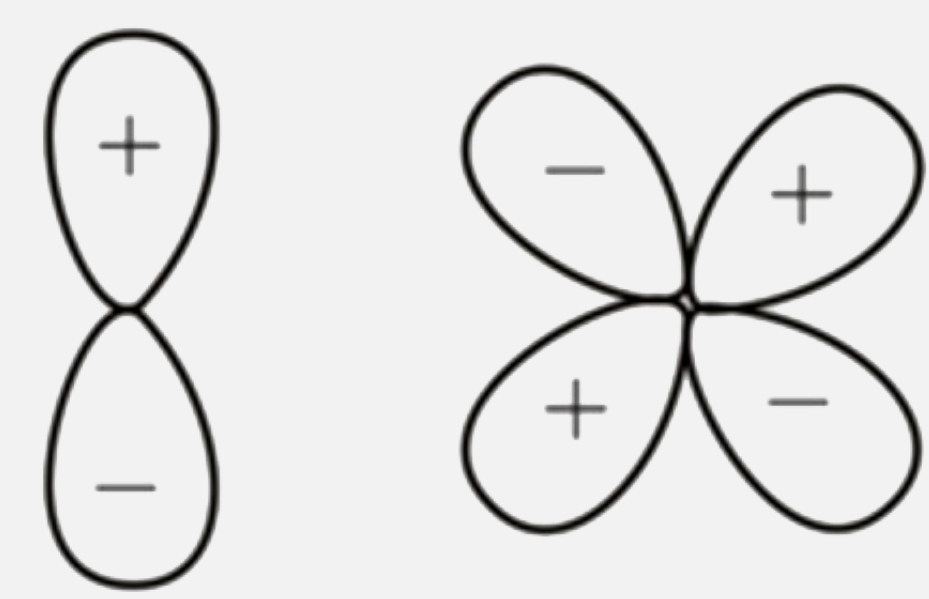A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 82-CHEMISTRY
- In Langumir's model of adosrption of a gas on a solid surface :
Text Solution
|
- Which of the following equation was suggested by de-Broglie?
Text Solution
|
- Which of the following gives rise to the formation of bond ?
Text Solution
|
- A metal oxide has the formula Z(2)O(3). It can be reduced by hydrogen ...
Text Solution
|
- Gypsum on heating above 437 gives
Text Solution
|
- An alkene on reductive ozonolysis gives two products A and B . When th...
Text Solution
|
- A mixture contains N(2)O(4) and NO(2) in the ratio 2:1 by volume. The ...
Text Solution
|
- Which of the following reactions will not give pure propane ?
Text Solution
|
- The noble gas which form interstitial compound with metals is
Text Solution
|
- The distance between an ocatahral and tetrahedral void in fcc lat...
Text Solution
|
- which of the following is the anhydride of NHO2 ?
Text Solution
|
- How many secondary and tertiary C - atoms are present in this compound...
Text Solution
|
- Pick out the incorrect statement for SO2.
Text Solution
|
- Consider the reactions (i) PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) (ii) ...
Text Solution
|
- If equal volumes of BaCI(2) and NaF solutions are mixed, which of thes...
Text Solution
|
- Which of the following reagents cannot be used to distinguish between ...
Text Solution
|
- Select the compound having maximum conductivity in aqueous medium.
Text Solution
|
- An alkane by molecular weight 72 upon chlorination gives one monochlor...
Text Solution
|
- Standard enthalpies of formation of O3,CO2,NH3 and HI are 142.2 , - 3...
Text Solution
|
- An electrolytic cell contains a solution of Ag(2)SO(4) and have platin...
Text Solution
|