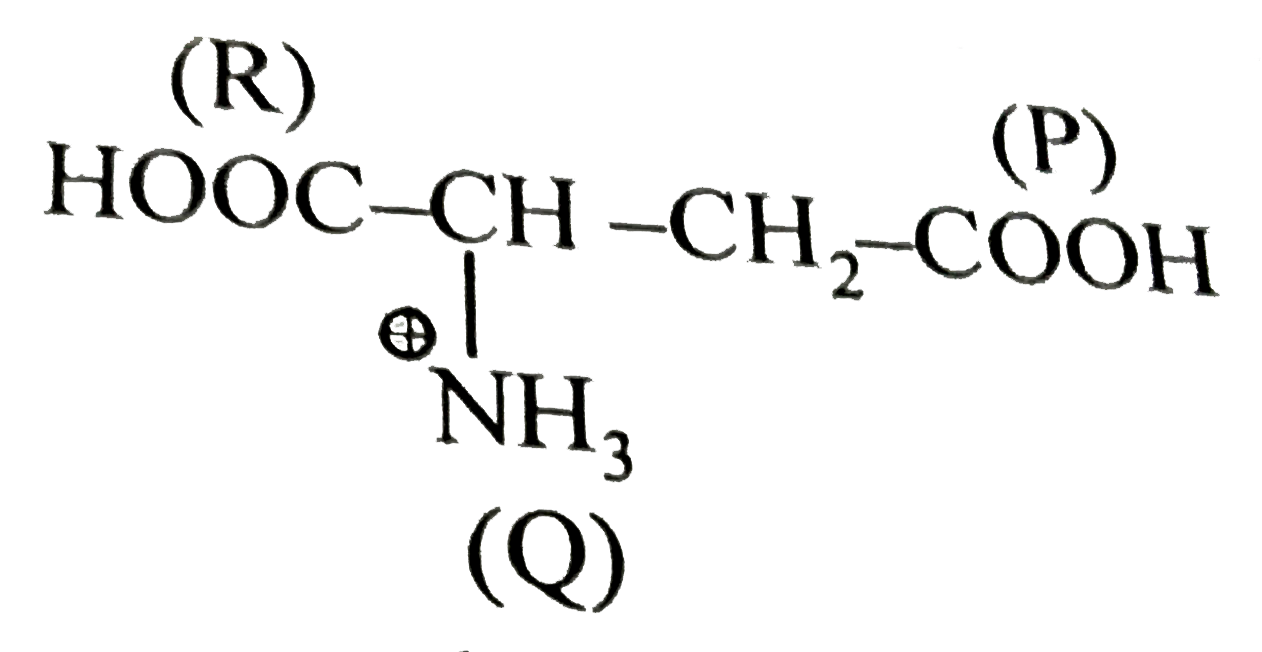A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 83-CHEMISTRY
- Presence of peroxide in ethers of old stock can be tested by first tre...
Text Solution
|
- A given mass of a gas expands from the state A to the state B by three...
Text Solution
|
- MnO(4)^(-) ions can be reduced in strongly alkaline medium to give
Text Solution
|
- A layer of chromium metal 0.25 mm thick is to be plated on an auto bum...
Text Solution
|
- Hydrolysis constants of two salts K(hA) and K(hB) of weak acids HA and...
Text Solution
|
- Artificial silk is a
Text Solution
|
- The pKa values for the three acidic group P,Q,R are 4.3, 9.7 and 2.2 r...
Text Solution
|
- Which can absorb large volume of hydrogen gas ?
Text Solution
|
- A reaction ArarrB , involes following mechanism : Step1 : Aoverset(...
Text Solution
|
- Which of the following statement is incorrect ?
Text Solution
|
- Identify the final product (Z) in the following sequence of reaction. ...
Text Solution
|
- Which of the following specie is diamagnetic in nature ?
Text Solution
|
- Aromatic aldehydes in the presence of cyanide ion as catalyst, are con...
Text Solution
|
- 40% (w/v) of NaCl solution (specific gravity = 1.12) is equivalent to
Text Solution
|
- Chromium is obtained by reducing connentrated chromite ore with :
Text Solution
|
- Which of the following species must have maximum number of electrons i...
Text Solution
|
- In the closest packing of is N atoms . There are
Text Solution
|
- Match the List I with List II and select the correct answer using the ...
Text Solution
|
- Consider the following statements: Phenyl diazonium salts form azo d...
Text Solution
|
- If the E(cell)^(@) for a given reaction has a positive value, then whi...
Text Solution
|