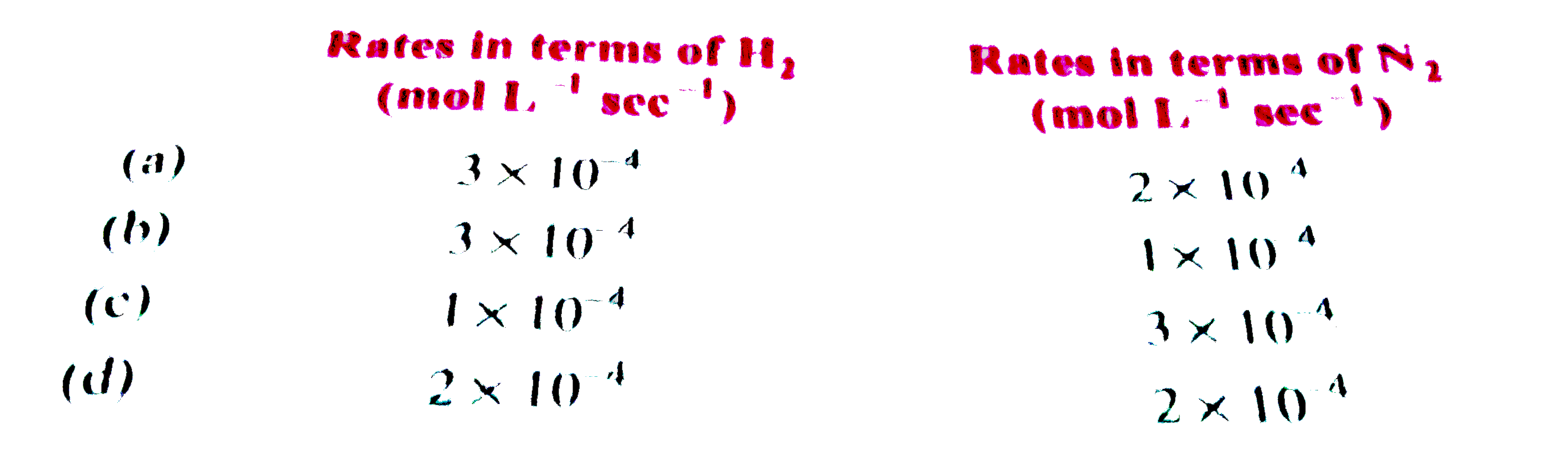A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 89-CHEMISTRY
- Ph-CHO+Ph-CHOoverset(Na(2)[Fe(CO)(4)])rarrPh-overset(O)overset("||")C-...
Text Solution
|
- Depletion of ozone layer causes
Text Solution
|
- Among the carbonates of alkali metals which one has highest thermal st...
Text Solution
|
- The efficiency of a hypothetical cell is about 84% which involves the ...
Text Solution
|
- An acid solution of pH=6 is diluted 1000 times, the pH of the final so...
Text Solution
|
- The number of aromatic structures possible for the molecular formula C...
Text Solution
|
- Which of the following alkenes is the most stable ?
Text Solution
|
- During micelle formation :
Text Solution
|
- The graph between log t(1//2) and log a at a given temperature is ...
Text Solution
|
- Consider the following reaction CH3-underset((A))(CH2)-O-H+CH3-underse...
Text Solution
|
- In the given reaction [C6H5-CH2-CH2-overset(o+)(N)(CH3)2-CH2-CH3]overs...
Text Solution
|
- Select correct statement for Cr. 6NH3.Cl3 and Cr.5NH2.Cl3
Text Solution
|
- In the reaction CH(3)-C-=C-Hoverset(CH(3)MgBr)toCH(4)+(A)underset((i...
Text Solution
|
- In the hober's process of ammonia manufacture, N(2)(g)+3H(2)(g) rarr...
Text Solution
|
- The period number and group number of "Tantalum" (Z=73) are respective...
Text Solution
|
- Which of the following will not form Grignard reagent with Mg/dry ethe...
Text Solution
|
- A certain sample of cuprous sulphide is found to have composition Cu(1...
Text Solution
|
- The product of the reaction CHO+(C6H5-CHCO)2Ooverset(C6H5-CH2COONa//D...
Text Solution
|
- Which one of the following behaves both as a nucleophile and an electr...
Text Solution
|
- Here the compound Y is
Text Solution
|