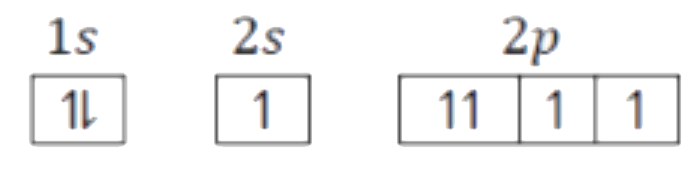
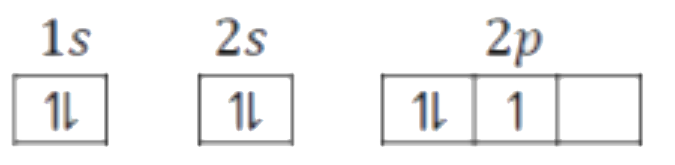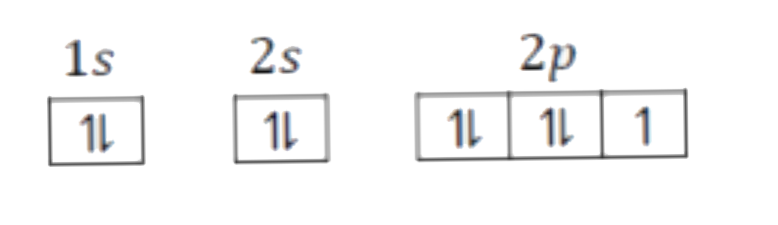A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 90-CHEMISTRY
- Which of the following 0.1 M complex compound solutions will hav...
Text Solution
|
- Which of the following is an amorphous substance ?
Text Solution
|
- Which of the following will violates Aufbau principle as well as Pauli...
Text Solution
|
- Hexachloroethane is also called
Text Solution
|
- In a reaction RCHO is reduced to RCH(3) usig amalgamated zinc and cenc...
Text Solution
|
- The products formed in the following reaction C(6)H(5)-O-CH(3)+HI ov...
Text Solution
|
- Terylene is a polymer obtianed from
Text Solution
|
- Aspirin is chemically :
Text Solution
|
- The IUPAC name of K4[Fe(CN)6] is
Text Solution
|
- For the combustion of n-octane C(8)H(18)+O(2)rarrCO(2)+H(2)O at 25^(...
Text Solution
|
- Which one is the electron deficient compound ?
Text Solution
|
- The IUPAC name of tertiary butyl chloride is
Text Solution
|
- Isomers which can be interconverted through rotation around a single b...
Text Solution
|
- An aqueous solution contain either Hg2^(2+) " or " Hg^(2+) the given s...
Text Solution
|
- 100 ml of 5 m H2SO4 of density 1 gm/ml is mixed with 100 ml of 8 m H2S...
Text Solution
|
- Benene diaxonium chloride in aqeous solution decomposes as : C6H5-N=N^...
Text Solution
|
- Which of the following has largest ionic size
Text Solution
|
- In NH3 solution of Zn^(2+),Zn^(2+) " form" [Zn(NH3)4]^(+2) In this sol...
Text Solution
|
- The reaction of , water gas (CO+H2)+H2 at 673 K, 300 atmosphere in pre...
Text Solution
|
- Beckmann's thermometer measures :
Text Solution
|