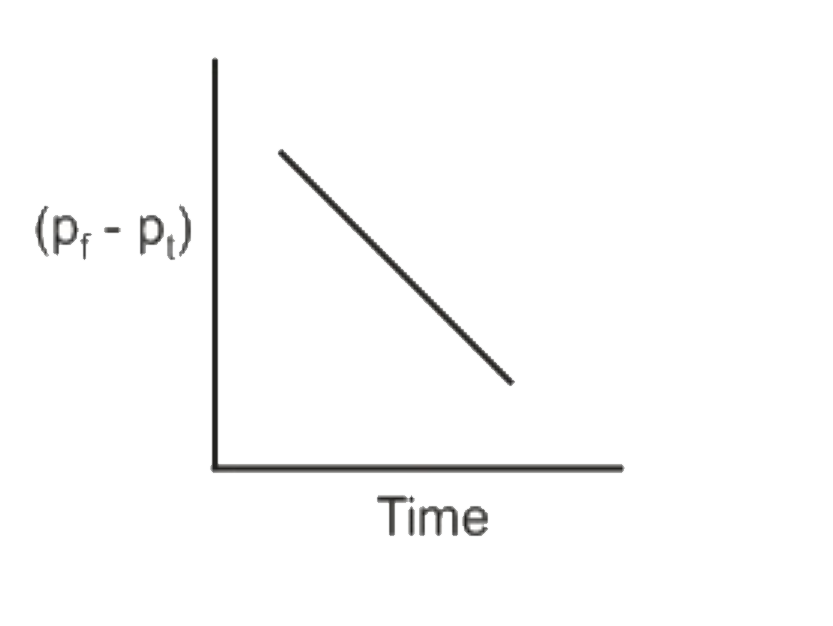
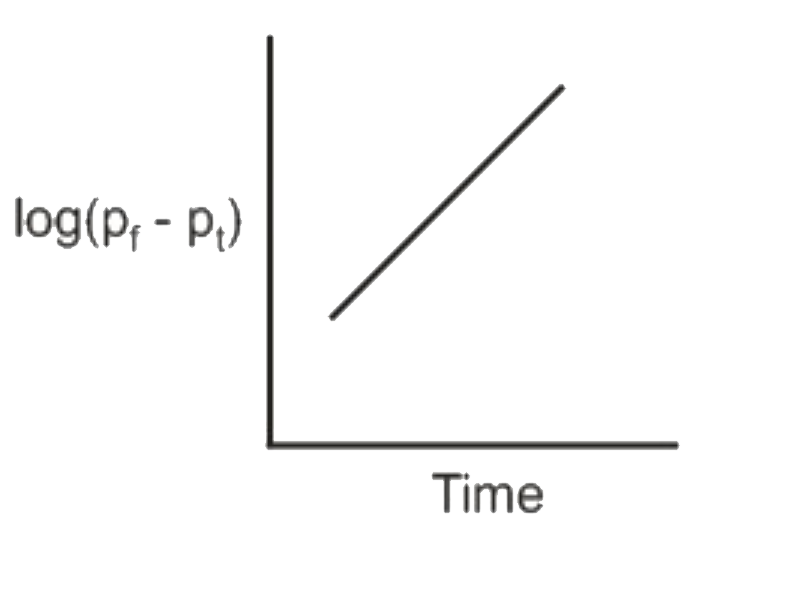
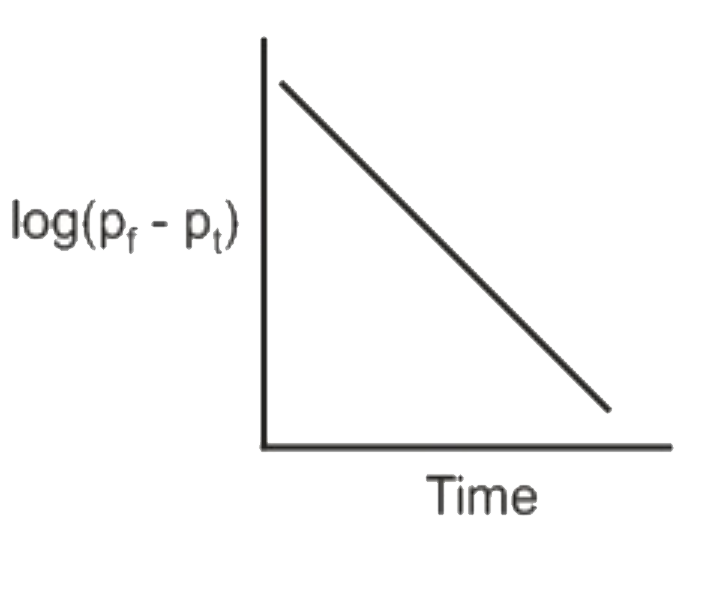
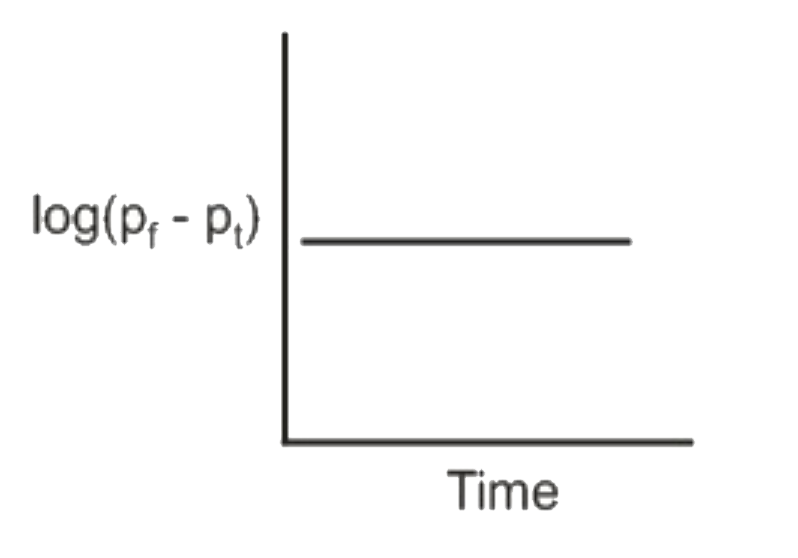
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 90-CHEMISTRY
- An aqueous solution contain either Hg2^(2+) " or " Hg^(2+) the given s...
Text Solution
|
- 100 ml of 5 m H2SO4 of density 1 gm/ml is mixed with 100 ml of 8 m H2S...
Text Solution
|
- Benene diaxonium chloride in aqeous solution decomposes as : C6H5-N=N^...
Text Solution
|
- Which of the following has largest ionic size
Text Solution
|
- In NH3 solution of Zn^(2+),Zn^(2+) " form" [Zn(NH3)4]^(+2) In this sol...
Text Solution
|
- The reaction of , water gas (CO+H2)+H2 at 673 K, 300 atmosphere in pre...
Text Solution
|
- Beckmann's thermometer measures :
Text Solution
|
- An element with atomic number 20 will be placed in which period of the...
Text Solution
|
- When dry chlorine gas is passed through silver chlorate heated to 90^@...
Text Solution
|
- The given reaction is an example of -
Text Solution
|
- The number average molecular mass and mass average molecular mass of a...
Text Solution
|
- The IUPAC name of the compound, CH2=underset(CH3)underset(|)C-CH2-C-=C...
Text Solution
|
- The dipole moment of H(2)O(2) is more than that of H(2)O but H(2)O(2) ...
Text Solution
|
- What is X in the nuclear reaction .(7)N^(14) + .(1)H^(1) rarr .(8)O^...
Text Solution
|
- Select the best reagent (s) to accomplish the following transformation
Text Solution
|
- The maximum possible number of hydrogen bonds a water molecule can for...
Text Solution
|
- The equilibrium constant K for the reaction 2HI(g) hArr H(2)(g)+I(2)(g...
Text Solution
|
- Which of the following pairs of compounds are position isomers?
Text Solution
|
- For the reaction N(2(g)) + O(2(g))hArr2NO((g)), the value of K(c) at 8...
Text Solution
|
- An organic compound having molecular mass 60 is found to contain C = 2...
Text Solution
|