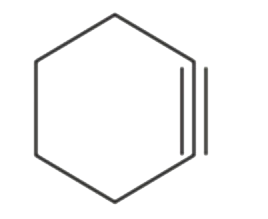
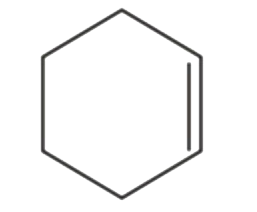
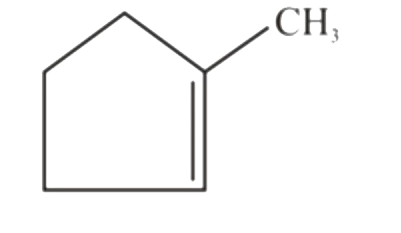
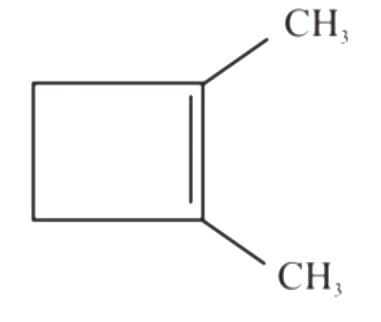
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET SET 96-CHEMISTRY
- The hybrid state and oxidation state of S in SF4 are respectively
Text Solution
|
- Mixture of volatile components A and B has a total vapour pressure (in...
Text Solution
|
- Compound A on oxidation with OsO4//NaHSO3 following by reaction with H...
Text Solution
|
- Consider the following sequence of reaction: Na+NH(3)(g) to [X]overs...
Text Solution
|
- An electron of a velocity 'x' is found to have a certain wavelength. T...
Text Solution
|
- Hydrogen molecules are
Text Solution
|
- In the given reaction C6H5-underset(O)underset(||)C-CH3overset(NH2OH//...
Text Solution
|
- Solubility of Zirconium phosphate Zr3(PO4)4 is 's' moles per litre. So...
Text Solution
|
- An element (atomic mass = 100 g//mol) having bcc structure has unit ce...
Text Solution
|
- Which one of the following compounds is least reactive with water?
Text Solution
|
- In [Cr(O(2))(NH(3))(4)H(2)O]Cl(2) oxidation number of Cr is +3 then ox...
Text Solution
|
- In which of the following solution the depression in freezing point is...
Text Solution
|
- Consider the following HOC-CH2-CH2-CH2CH2OH overset(overset(o+)H"or")u...
Text Solution
|
- What is the sign of DeltaG^@ and the value of K an electrochemical cel...
Text Solution
|
- Consider the following compounds Order of basicity of these compo...
Text Solution
|
- The products of the reaction will be C6H6+CH3-CH2-CH2-Broverset("Anh...
Text Solution
|
- Arrange the following as indicated. CO2,N2 O5, SiO2 and SO3 in the o...
Text Solution
|
- An open vessel at 27^(@)C is heated until 3//5 of the air in it is exp...
Text Solution
|
- In the given reaction C6H5-CH=CH-CH3+HCl rarr[X] [X] will be
Text Solution
|
- The resistance of 0.01 N solution at 25^(@)C is 200 ohm. Cell constant...
Text Solution
|