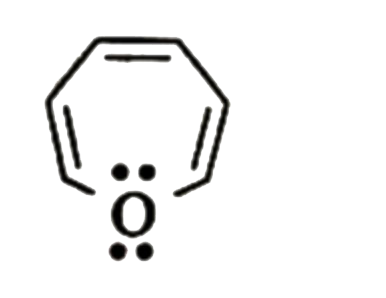
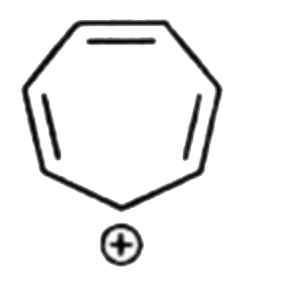
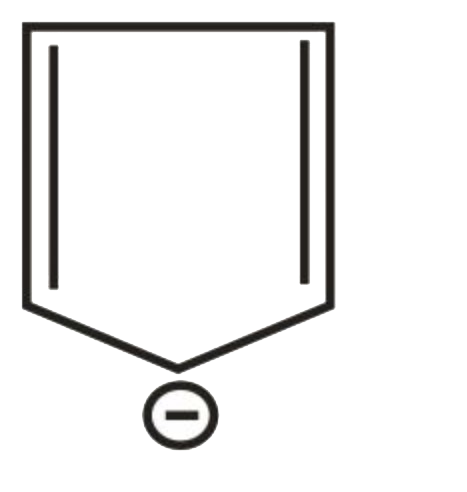
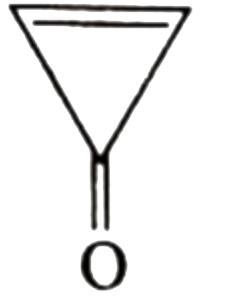
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA NEET TEST 101-CHEMISTRY
- Mole ratio of Fe in FeO,Fe2O3 and Fe3O4 samples of equal weights is
Text Solution
|
- CS2 and SO3 react to produce
Text Solution
|
- Which one of the following compound is not a planar ?
Text Solution
|
- An ideal gas of certain mass is heated in a small vessel and then in a...
Text Solution
|
- In the given reaction overset(Br)overset(|)(CH2)-CH=CH2overset(NaNH2//...
Text Solution
|
- Average oxidation number of carbon in C3O2,Mg2C3 are respectively.
Text Solution
|
- The change in entropy, Delta S is positive for an endothermic reaction...
Text Solution
|
- N2+H2rarr[X](g)overset(CO2,"Pressure")rarr[Y]overset("Heat")rarr[Z]+H2...
Text Solution
|
- In the given reaction CH3-CH2-overset(Br)overset(|)(CH)-CH=CH2overset(...
Text Solution
|
- BeO+CrarrCO+Xoverset(H2O)rarrBe(OH)2+Y; X and Y in the above sequenc...
Text Solution
|
- 2 g molecule of PCl5 are heated in a closed vessel of two litre capaci...
Text Solution
|
- ltBrgt Match the physical changes in List-I with their relations given...
Text Solution
|
- The given reaction is CH3-CH=CH-CH2OHoverset(HBr)rarrCH3-overset(Br)ov...
Text Solution
|
- The ligand shown here is
Text Solution
|
- Compound A on oxidation with not KMnO4//bar(O)H given two compound CH...
Text Solution
|
- In an irreversible process taking place at constant T and P and in whi...
Text Solution
|
- Each of the three metals X,Y and Z were put in turn into aqueous solut...
Text Solution
|
- SCl2 is the best known dihalice of sulphur, hybrid state of sulphur in...
Text Solution
|
- Which of the following is correct for the velocity of electron ?
Text Solution
|
- Which oxide of carbon is obtained when K(4)[Fe(CN)(6)] is warmed with...
Text Solution
|