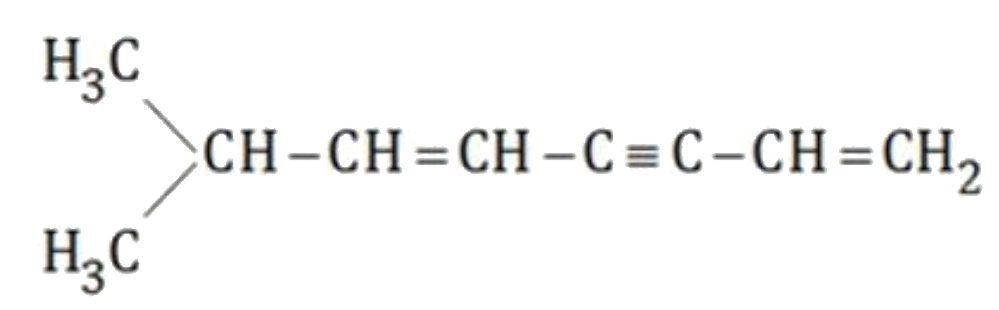A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 74-CHEMISTRY
- For the reaction, 2X(3)hArr 3X(2), the rate of formation of X(2) is
Text Solution
|
- 25 mol of formic acid (HCO(2)H) is dissolved in enough water to make o...
Text Solution
|
- Consider the given statement about the molecule 1. Three carbon a...
Text Solution
|
- Among the following, the group of molecules that undergoes rapid hydro...
Text Solution
|
- Two inflated ballons I and II (thin skin) having volume 600 mL and 150...
Text Solution
|
- The compound given below on heating gives P (Major product)
Text Solution
|
- What mass of H(2)C(2)O(4). 2H(2)O (mol.mass=126) should be dissoved in...
Text Solution
|
- The complex that exists as a pair of enantiomers is
Text Solution
|
- Given that standard potential for the following half - cell reaction a...
Text Solution
|
- The reaction of acetaldehyde and HCN, followed by complete acid hydrol...
Text Solution
|
- The hydrocarbon of molecular mass 72 gives a single monochloride and t...
Text Solution
|
- For the process, 1 Ar (300 K, 1 bar) rarr 1 Ar (200 K, 10 bar), assumi...
Text Solution
|
- Consider the reaction, (CH(3))(3)C-underset(CH(3))underset("| ")"CH...
Text Solution
|
- Which of the following is not correct?
Text Solution
|
- Pick out incorrect statement
Text Solution
|
- The pressure of a mixture of equal weight of two gases of mol wt. 4 an...
Text Solution
|
- How many of these metals can be purified by vapour phase refining Ni...
Text Solution
|
- Consider the following compounds : CH(4),CH(3)-CH(3), CH(3)OH, CH(3)...
Text Solution
|
- The wave number of first line of Balmer series of hydrogen is 1520m^(-...
Text Solution
|
- How may optical isomers are possible for CH(3)overset(H)overset("|")...
Text Solution
|