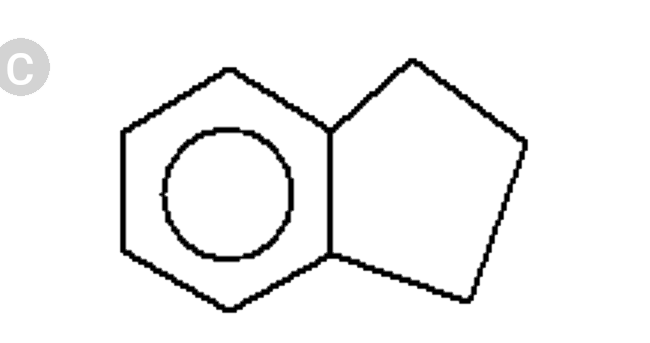
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 78-CHEMISTRY
- The aqueous 0.01 Molal solution of [Cr(NH(3))(6)](2)[Co(NH(3))(NO(2))(...
Text Solution
|
- For (A)+K(2)CO(3)+air overset(Heat)rarr(B) (B)+CI(2)rarr(C)pink Wh...
Text Solution
|
- Which of the following has smallest radius?
Text Solution
|
- If r(1) is the radius of first orbit of hydrogen atom, then the radii ...
Text Solution
|
- N2 and O2 are converted to mono cations N2^+ and O2^+ respectively, wh...
Text Solution
|
- In the carbylamine reaction, R-X converted R-Y via the intermediate Z,...
Text Solution
|
- The equilibrium constant for the reaction H(2)O(g)+CO(g)hArrH(2)(g)+CO...
Text Solution
|
- Ammonia forms the complex [Cu(NH3)4]^(2+) with copper ions in alkaline...
Text Solution
|
- Sodium carbonate is prepared by Solvay process. Which of the following...
Text Solution
|
- The reaction of p-HOC(6)H(4)COOH with excess Br(2) forms
Text Solution
|
- Melamine polymer is a copolymer of
Text Solution
|
- When an inorganic compound (X) having 3c - 2e as well as 2c - 2e bonds...
Text Solution
|
- Maltose on hydrolysis gives
Text Solution
|
- Which product will be obtained by Gridnard reaction, when Formaldehyde...
Text Solution
|
- Consider the following sequence of reactions and identify the final pr...
Text Solution
|
- An exothermic reaction, A rarr B, has an activation energy of "15 kcal...
Text Solution
|
- The enolic form of given compound contians CH(3)-CH(2)-overset(O)ove...
Text Solution
|
- A certain buffer solution sontains equal concentration of X^(-) and HX...
Text Solution
|
- In cyclic structure of (SO(3))(x) if x is number of SO(3) moleculles i...
Text Solution
|
- If number of lone pairs on central atom in XeF(2), XeF(4) and XeF(6) a...
Text Solution
|