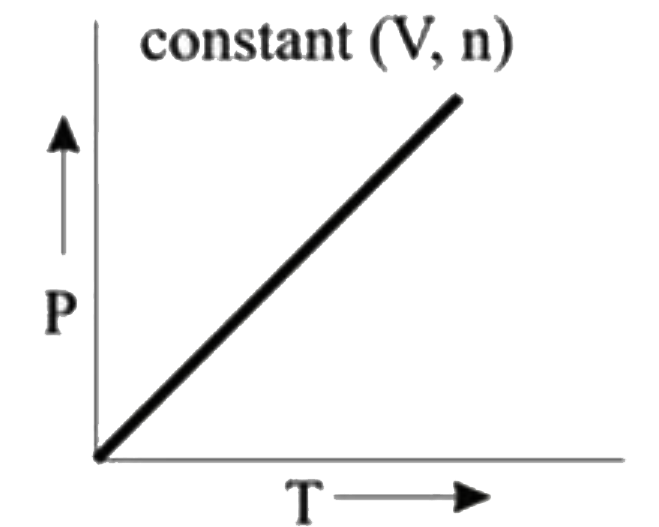
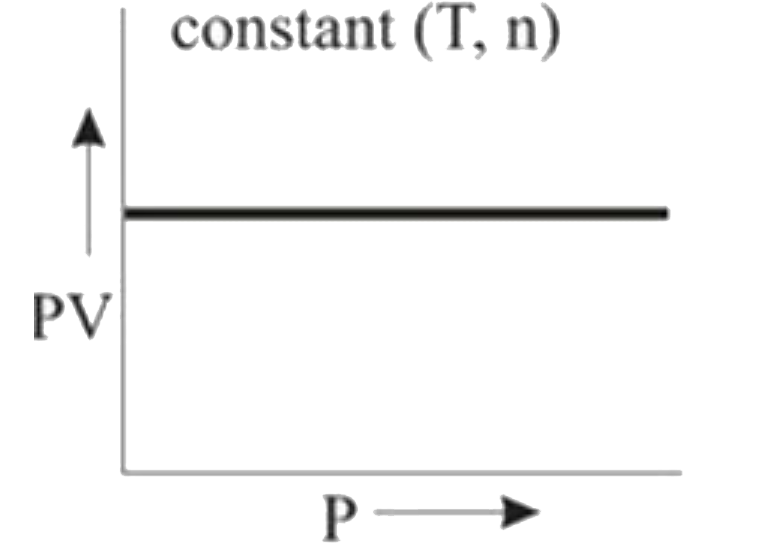
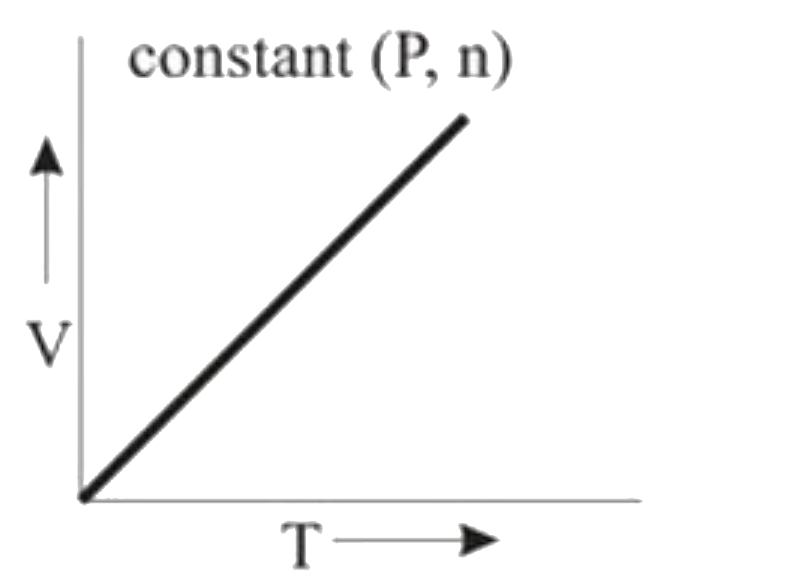
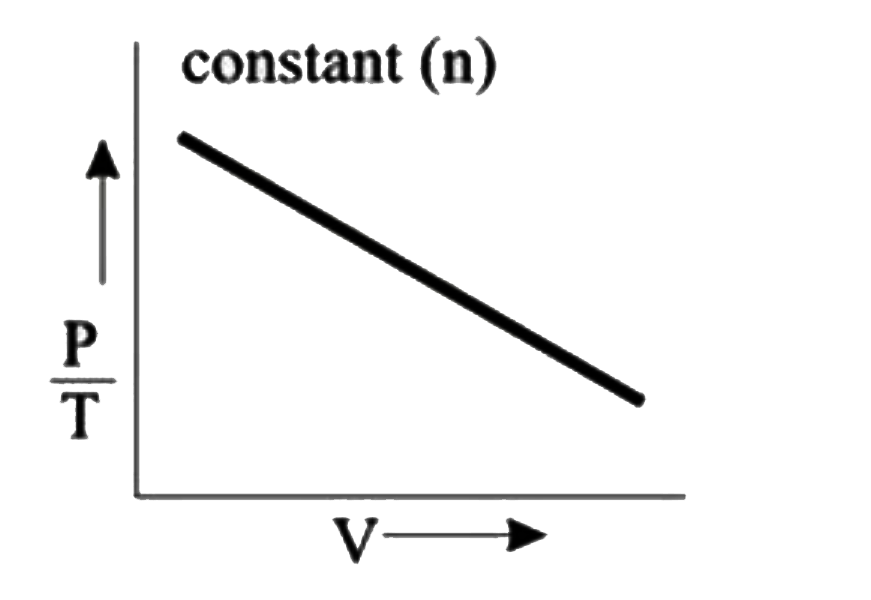
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 80-CHEMISTRY
- Conectrated aqueous sodium hydroxide can be a separated mixture of
Text Solution
|
- Consider the following substances 1. CH(3)C-= C CH(3) 2. CH(3)CH(...
Text Solution
|
- Which of the following graphs show most significant deviation from ide...
Text Solution
|
- Which of the following will be oxidised by HIO(4)? (A) R-underset(O)...
Text Solution
|
- The freezing point of a 0.08 molal solution of NaHSO^(4) is -0.372^(@)...
Text Solution
|
- Among the following the one that gives positive iodoform test upon rea...
Text Solution
|
- Potassium ferrocyanide is used in the detection of
Text Solution
|
- 1 g of .(79)Au^(198) (t(1//2) = 65 hr) decays by beta-emission to prod...
Text Solution
|
- The efficiency of the reversible cycle shown in the figure will be
Text Solution
|
- Ester containing alpha- hydrogens undergo self condensation in presenc...
Text Solution
|
- Which of the following is most likely structrure of CrCI(3).6H(2)O if ...
Text Solution
|
- X=5Å Y=8Å Molar mass of solid ="259.8 g mol"^(-1) A solid crysta...
Text Solution
|
- Calculate the cell potential of following cell Pt(s)|H(2)(g)"(0.1 bar...
Text Solution
|
- Identify the final product (z) in the following sequence of reactions ...
Text Solution
|
- Some drugs interact with enzymes and make the biologically inactive. S...
Text Solution
|
- Derivative of nitrogen (III) act as
Text Solution
|
- Which of the following statements is incorrect?
Text Solution
|
- Reduction of hexose A (molecular formula C(6)H(12)O(6)) with sodium bo...
Text Solution
|
- A compound with molcular formula C(4)H(10)O(3). is converted by the ac...
Text Solution
|
- How many of these molecules get dimerise by 3c - 4e bonds BeCl(2), A...
Text Solution
|