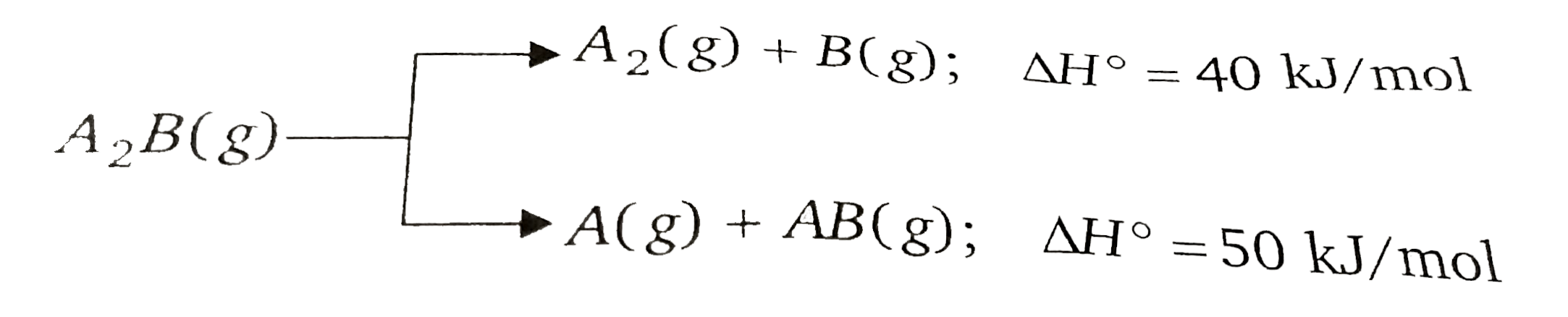A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 81-CHEMISTRY
- D is
Text Solution
|
- Three isothermal plots (P versus V) A, B and C are plotted at three te...
Text Solution
|
- Substance A(2)B(g) can undergoes decomposition to form two set of prod...
Text Solution
|
- overset(NaBH(4))(leftarrow) PhCH=CH-CHOunderset(2. H(3)O^(+))overset(1...
Text Solution
|
- (CH(3))(2)C=CHCOCH(3) can be oxidised to (CH(3))(2) C=CHCOOH by
Text Solution
|
- Pick out the incorrect statement.
Text Solution
|
- In a copper voltmeter, mass deposite in 30 seconds is 'm' gram. If the...
Text Solution
|
- The final product C in the following reaction is
Text Solution
|
- 0.2 gm sample of benzoic acid C(6)H(5)COOH is titrated with 0.12 M Ba(...
Text Solution
|
- (CH(3))(2)CHOH overset("Mild")underset("oxidation[O]")rarr (X) overset...
Text Solution
|
- Which of the following compound will given yellow precipitate on ...
Text Solution
|
- Which of the following oxyacids acts as most reducing agent?
Text Solution
|
- Consider the following acids 1. MeCH(2)COOH 2. Me(2)CHCOOH 3. Me...
Text Solution
|
- The plot of (1)/(Y(A))" Vs "(1)/(x(A))((1)/(Y(A))" on y - axis") where...
Text Solution
|
- The transition elements are more metallic then p - block elements beca...
Text Solution
|
- Which of the following reaction are possible ? (1) ArN(2)^(+) + CuBr...
Text Solution
|
- Which of the following order is correct for the property mentioned in ...
Text Solution
|
- How many of these molecules are diamagnetic and have bond order more t...
Text Solution
|
- (x)/(20)M concentration of H^(+) ion must be maintained in a saturate ...
Text Solution
|
- How many moles of HI consumed in above reaction?
Text Solution
|