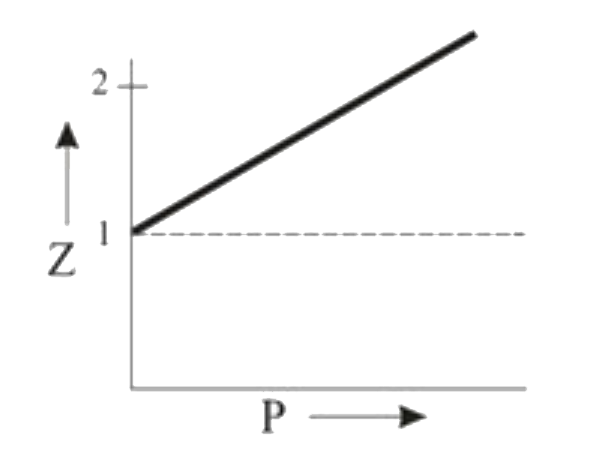
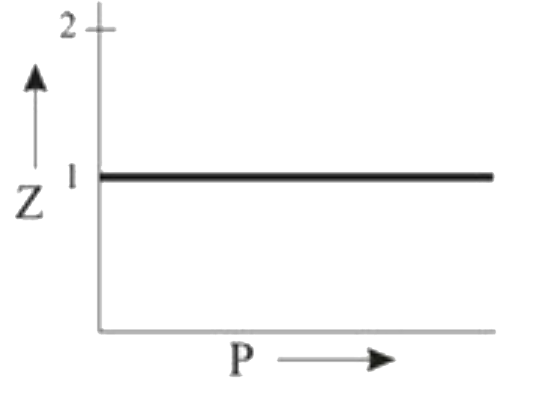
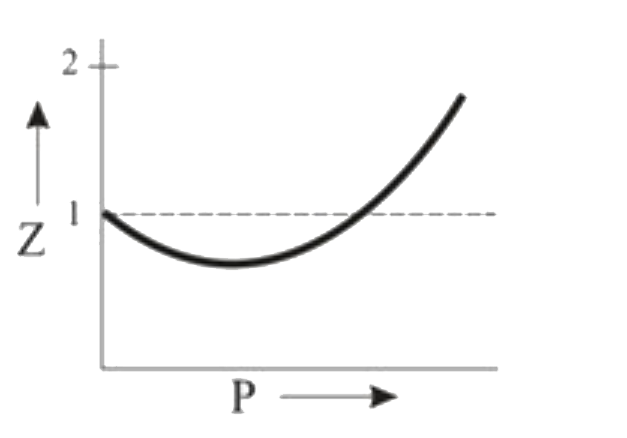
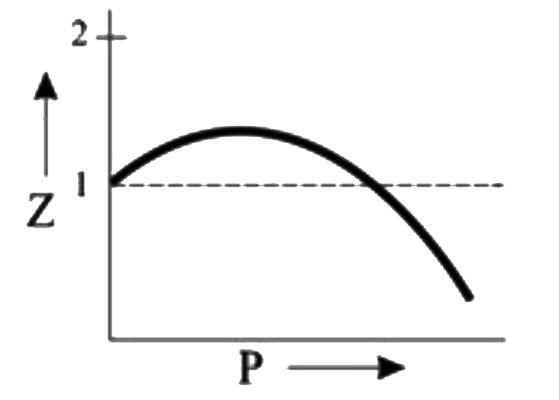
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 82-CHEMISTRY
- Pick out the incorrect statement
Text Solution
|
- The optical rotation of the alpha-form of a pyranose is +150.7^(@), th...
Text Solution
|
- Give the produst when an excess of PhMgBr//H^(+) reacts with dimethyl ...
Text Solution
|
- What is the freezing point of a solution containing 8.1g Br in 100g wa...
Text Solution
|
- The reducing power of a metal depends on various factors. Suggest the ...
Text Solution
|
- The solubility product of BaCrO(4) is 2.4xx10^(-10)M^(2). The maximum ...
Text Solution
|
- The compund 'A' is
Text Solution
|
- A metal M and its compound can give the following observable changes i...
Text Solution
|
- End product C in above reaction is
Text Solution
|
- Mechanism of a hypothetical reaction X(2) + Y(2) rarr 2XY is given b...
Text Solution
|
- In the following reaction, which of the following steps is wrong ?
Text Solution
|
- Specify the coordination geometry around and the hybridisation of N an...
Text Solution
|
- For a given reaction DeltaH="35.5 k Jmol"^(-1) and DeltaS="83.6 Jk"^(-...
Text Solution
|
- A six coordination complex of formula CrCl(3)*6H(2)O has green colour....
Text Solution
|
- Which of the following represents a plot of compressibility factor (Z)...
Text Solution
|
- Equilibrium constant for reaction NH(4)OH(aq)+H^(+)(aq)hArr NH(4)^(+)(...
Text Solution
|
- How many Cl - atoms are present in Bithional added in soaps for (antis...
Text Solution
|
- If sucrolose in nxx100 times of more sweet than Aspartame. What is the...
Text Solution
|
- How many of these days acids are mobobasic in nature? H(2)S, H(2)O(...
Text Solution
|
- In Melamine the total number of N- atoms having sp^(2) hybridisation a...
Text Solution
|