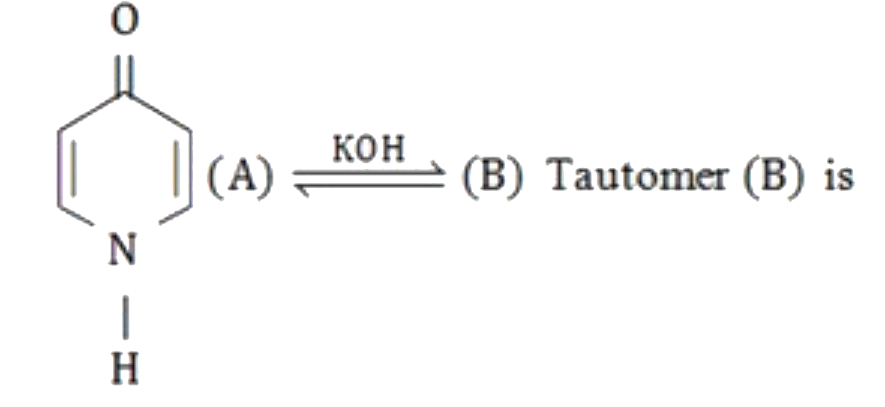
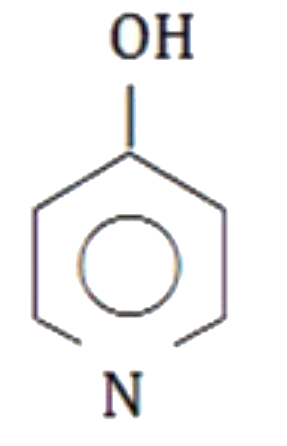
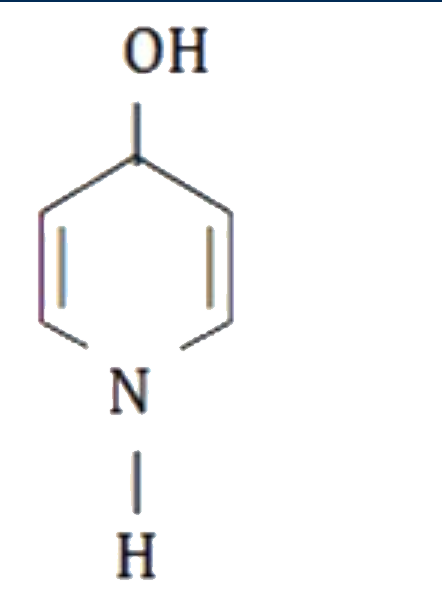
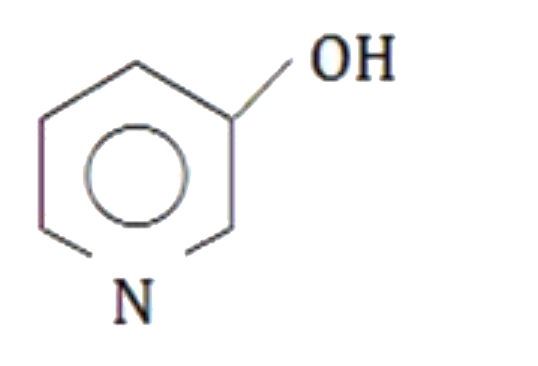
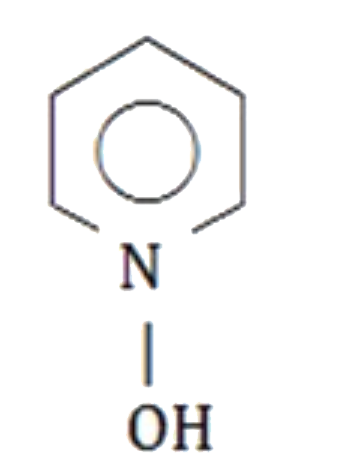
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
NTA MOCK TESTS-NTA JEE MOCK TEST 83-CHEMISTRY
- The correct order of boiling point is
Text Solution
|
- In the reaction , 4A+2B+3CtoA(4)B(2)C(3) what will be the number of ...
Text Solution
|
- Complete the following reaction
Text Solution
|
- An alpha- paticle approaches the target nucleus of copper (Z = 29) is ...
Text Solution
|
- [Fe(H(2)O)(6)]^(2+) and [Fe(CN)(6)]^(4-) differ in
Text Solution
|
- Ten moles of an ideal gas are filled in a closed vessel. The vessel ha...
Text Solution
|
- Which of the following is not a resonance structure of the other?
Text Solution
|
- Nitrogen dioxide can not be obtained from
Text Solution
|
- The S-S bond energy is if DeltaH(f)^(@)(E(t)-S-E(t))=-147kJ//mol,Delta...
Text Solution
|
- The major product in the reaction is
Text Solution
|
- BX(3)+NH(3) overset(B.T.) to BX(3) *NH(3)+Heat of adduct formation (De...
Text Solution
|
- A 250.0 mL sample of a 0.20M Cr^(3+) is electrolysed with a current of...
Text Solution
|
- Which of the following is least reactive towards S(N)1?
Text Solution
|
- For a second order reaction, 2Ararr Products, a plot of log t(1//2) v...
Text Solution
|
- Consider the following metallurgical processes: (I) Heating impure m...
Text Solution
|
- (A) overset(HCI+ZnCI(2))larr Me(3)C-CH(2)OH overset(SOCI(2))underset(P...
Text Solution
|
- The silicate anion in the mineral kinoite is a chain of three SiO(4) t...
Text Solution
|
- The compound (X) is
Text Solution
|
- What will be the pH of a solution formed by mixing 40cm^(2) of 0.1 M H...
Text Solution
|
- Which of the following compounds is likely to be a product Y in this c...
Text Solution
|