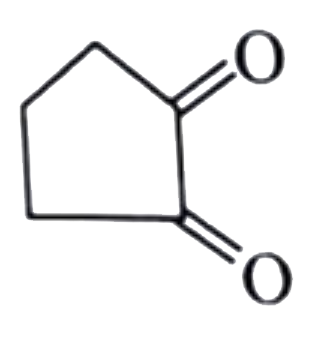
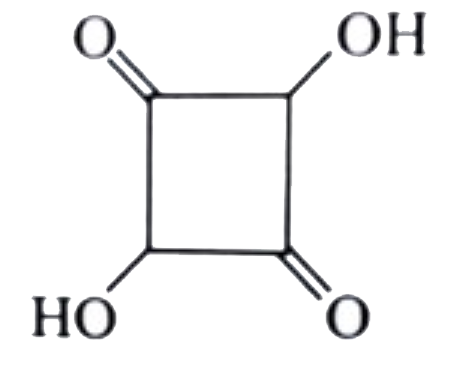
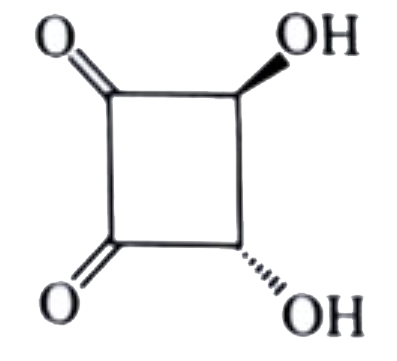
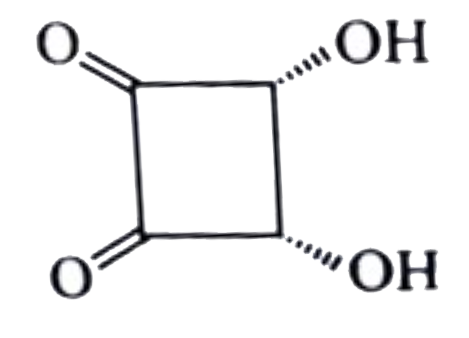
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 85-CHEMISTRY
- Heat energy absorbed by a system in going through a cyclic process sho...
Text Solution
|
- 2-Mehtylbut-1-ene reacts wth mercuric acetate in presence of water to ...
Text Solution
|
- Among the following the optically inactive compound is :
Text Solution
|
- Which of the group element does not form M(III) iodide?
Text Solution
|
- A quantity of electrcity required to reduce 12.3 g of nitrobenzene to ...
Text Solution
|
- Silver ions are added to a solution with [Br^(-)]=[Cl^(-)]=[CO(3)^(2-)...
Text Solution
|
- In which of the following molecular species sigma- dative bond is pres...
Text Solution
|
- There is S-S bond in
Text Solution
|
- Conpound (A) underset(HIO(4))overset(2 mol of)(rarr) 2 mol of glyoxali...
Text Solution
|
- Compound PdCl(4). 6H(2)O is a hydrated complex, 1 molal aqueous soluti...
Text Solution
|
- Which of the following metal nitrate produces gaseous product when rea...
Text Solution
|
- Product 'C' is
Text Solution
|
- If K(1) and K(2) are respective equilibrium constants for two reactio...
Text Solution
|
- An organic compound (A) with molecular formula C(7)H(8)O dissolves in ...
Text Solution
|
- In a compound XY(2) O(4) , oxide ions are arranged in CCP and cations ...
Text Solution
|
- How many Cl - atoms are present in Bithional added in soaps for (antis...
Text Solution
|
- For a first order reaction, if the time taken for completion of 50% of...
Text Solution
|
- How many alkenes possible by the dehydration of
Text Solution
|
- The oxidation number of Mn in the product of alkaline oxidative fusion...
Text Solution
|
- How many of these molecules can have any type of hydrogen bonding. G...
Text Solution
|