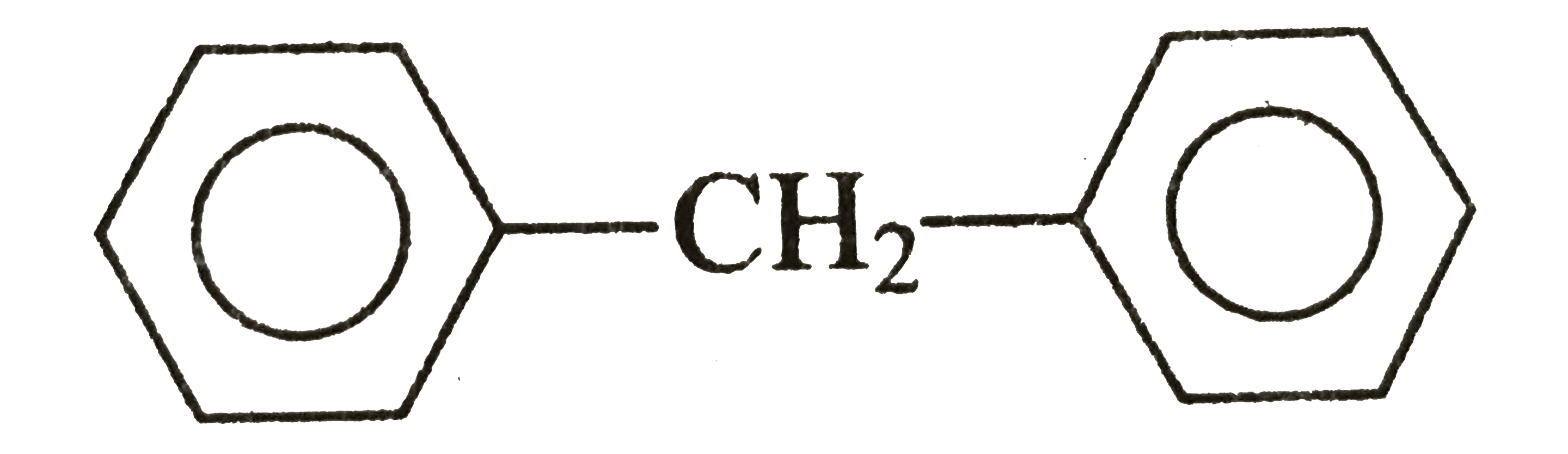Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 88-CHEMISTRY
- Determine the value of equilibrium constant for the reaction 2Br^(-)(a...
Text Solution
|
- Consider the following reaction [X] and [Y] respectively be
Text Solution
|
- The reaction sequence C(6)H(5)-CH=CH-Choverset([X])rarr C(6)H(5)-CH(...
Text Solution
|
- The correct increasing order of extent of hydrolysis is
Text Solution
|
- Let a fully charged lead storage battery contains 1.5 L 5 M H(2)SO(4)....
Text Solution
|
- Three sparingly soluble salts that have same solubility products as gi...
Text Solution
|
- Correct sequence of CO bond order in given compounds is: (P) Fe(CO)(...
Text Solution
|
- Give the correct order of initials T or F for following statements. Us...
Text Solution
|
- In the given reaction CH(2)-cH(2)-overset(O)overset(||)C-CH(2)COOC(2...
Text Solution
|
- Volume of 0.1 M K(2)Cr(2)O(7) required to oxidize 35 mL of 0.5 M FeSO(...
Text Solution
|
- The correct order of increasing solubility in water is:
Text Solution
|
- In the given reaction sequence C(6)H(5)CHO+CH(3)-NO(2)overset((i)NaO...
Text Solution
|
- For (A)+K(2)CO(3)+air overset(Heat)rarr(B) (B)+CI(2)rarr(C)pink Wh...
Text Solution
|
- Consider the following carbanions (i) CH(3)- overset(Ө)CH(2)" "(ii...
Text Solution
|
- The degree of dissociation 'alpha' of the reaction N(2)O(4)(g)hArr 2...
Text Solution
|
- The wave function orbital of H-like atoms is given as under psi(2s) ...
Text Solution
|
- At 273 K one atm, 'a' litre of N(2)O(4) decomposes to NO(2) as : N(2...
Text Solution
|
- The energy released during conversion of million atoms of iodine in ga...
Text Solution
|
- The Henry's law constant for the solubility of N(2) gas in water at 29...
Text Solution
|
- How many structural isomers are possible when one of the hydrogen in ...
Text Solution
|