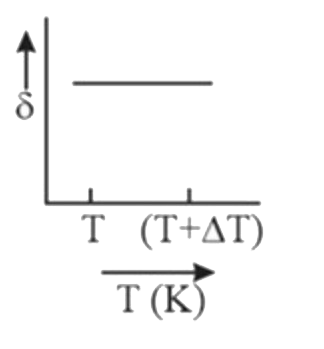
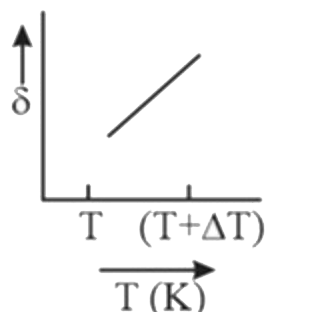
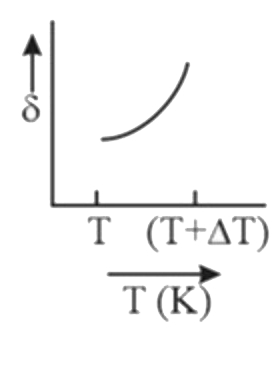
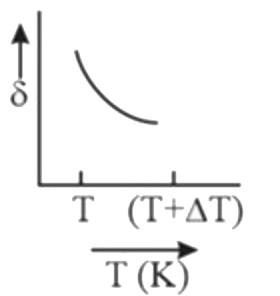A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 89-CHEMISTRY
- The CI - C - CI angle in 1, 1, 2, 2, tetrachloroethone and tetrachloro...
Text Solution
|
- An ideal gas is initially at temperature T and volume V. Its volume is...
Text Solution
|
- When photons of energy 4.25eV strike the surface of a metal A, the eje...
Text Solution
|
- A red coloured mixed oxide (X) on treatment with conc. HNO(3) gives a ...
Text Solution
|
- The Compound (A) and (B) in the equation given above are
Text Solution
|
- Product of the following reaction is
Text Solution
|
- An inorganic compound (X) made up of two most occurring elements in th...
Text Solution
|
- If P^(@) and P(S) are the vapour pressure of the solvent and solution ...
Text Solution
|
- Predict the major product P in the following reaction.
Text Solution
|
- Compounds (A) and B are treated with dilute HCl separately. The gases ...
Text Solution
|
- Equal amount of an RCl(C4 H9Cl) is reacted at the same temperature wit...
Text Solution
|
- What is the product of the following reaction?
Text Solution
|
- At pH = 2, E(("Quinhydrone"))^(@) = 1.30 V, E("Quinhydrone") will be :
Text Solution
|
- The net work done through a series of changes reported is figure at th...
Text Solution
|
- A solid AB has NaCl type structure with edge length 580.4 pm. Then ra...
Text Solution
|
- The number of geometrical isomers of [Co(NH(3))(3)(NO(2))(3)] are
Text Solution
|
- Product (X) is
Text Solution
|
- Euchlorine is
Text Solution
|
- Product (A) is
Text Solution
|
- What are the products formed in the reaction of xenon hexa fluoride wi...
Text Solution
|