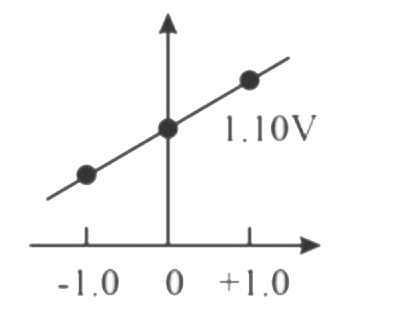A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 92-CHEMISTRY
- Which formula does not correctly represent the bonding capacity of the...
Text Solution
|
- Each of the compounmds Pt(NH(3))(6)Cl(4), Cr(NH(3))(6)Cl(3) and K(2)Pt...
Text Solution
|
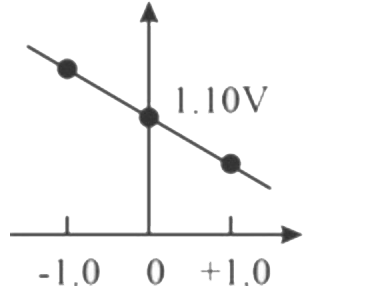
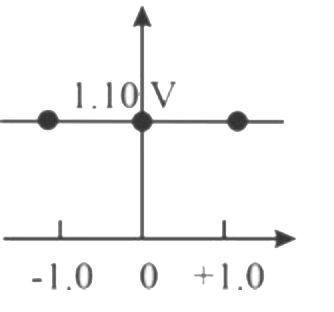
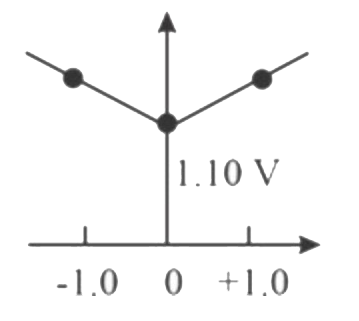
- Which graph correctly correlates E("cell") as a function of concentrat...
Text Solution
|
- Arrange the following compounds in order of their reactivity towards L...
Text Solution
|
- Compound (C ) is
Text Solution
|
- The elevation in boiling point of a solution dT(b) is related with mol...
Text Solution
|
- Given the decreasing order of reactivity of the following compounds wi...
Text Solution
|
- Consider the following reaction and Identify (B)
Text Solution
|
- What is the equation form of Langmuir adsorption isotherm undre high p...
Text Solution
|
- Which is the correct order of decreasing boiling point of azeotropic m...
Text Solution
|
- In the following reaction, the final product can be prepared by two pa...
Text Solution
|
- Point out the incorrect statement among the following?
Text Solution
|
- What will be the order of reaction and rate constant for a chemical ch...
Text Solution
|
- In the Hoope’s process for refining of aluminium, the fused materials ...
Text Solution
|
- The oxidation number is changed in which of the following case?
Text Solution
|
- The temperature coefficient of e.m.f of a cell can be given by :
Text Solution
|
- Given the major product of the following reaction.
Text Solution
|
- How many unit cell are present in a cubic-shaped ideal crystal of NaCl...
Text Solution
|
- Which of the following pairs of compounds are more stable?
Text Solution
|
- A spherical ballon of 21 cm diameter is to be filled with H(2) at NTP ...
Text Solution
|