A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 95-CHEMISTRY
- In Mayer's relation: C(P)-C(V)=R 'R' stands for:
Text Solution
|
- Products of the reaction which is given below will be CH(3)-underset...
Text Solution
|
- (Z) -3- bromo -3- hexene when treated with CH(3)O^(-)" in "CH(3)OH giv...
Text Solution
|
- Two oxides of nitrogen, NO and NO(2) are allowed to react together at ...
Text Solution
|
- A carbon compound contains 12.8% of carbon, 2.1% of hydrogen and 85.1%...
Text Solution
|
- Calcium lactate is a salt of weak organic acid and strong base represe...
Text Solution
|
- The octahedral complex/complex ion which shown both facial and meridio...
Text Solution
|
- The correct code for stability, of oxidation states for given cations ...
Text Solution
|
- The most appropriate sequence of the reactions for carrying out the fo...
Text Solution
|
- pH of 0.1 M monobasic acid is found to be 2 . Hence its osmotic pressu...
Text Solution
|
- The reduction of an oxide by aluminium is called
Text Solution
|
- Match list - I with list - II and select the correct answer using the ...
Text Solution
|
- Which of the following is second ion is more stable than the first
Text Solution
|
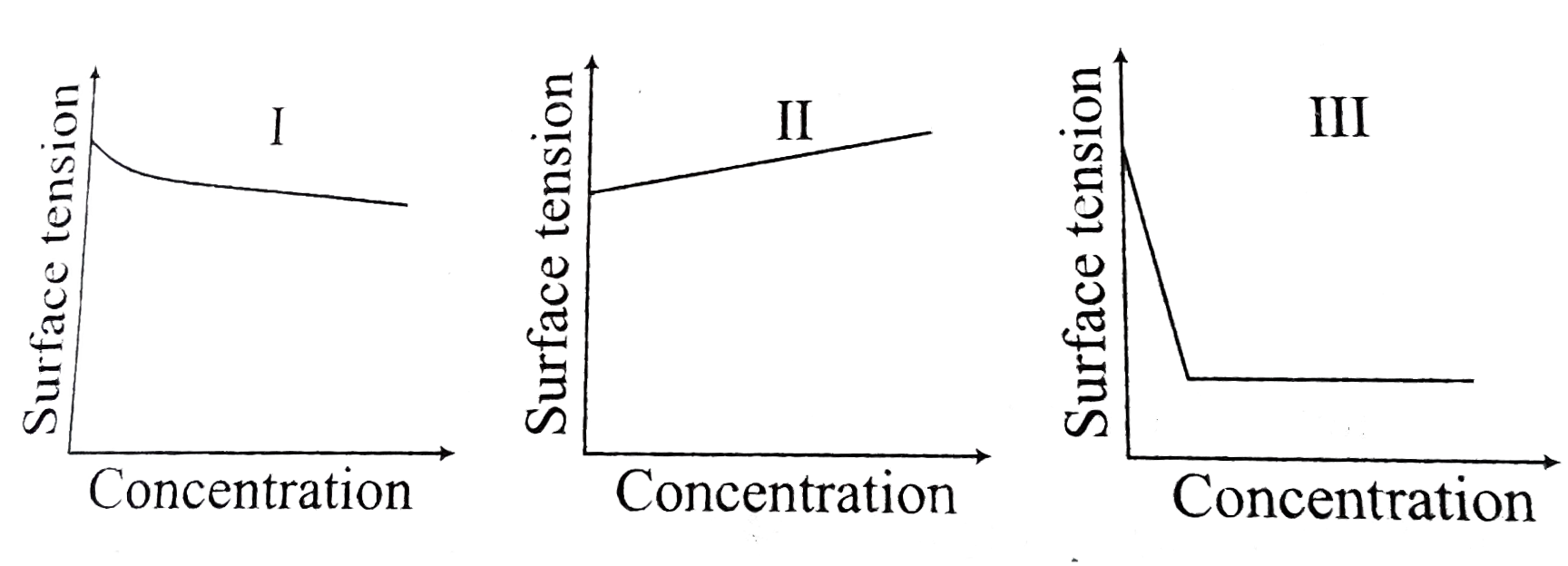
- The equalitative sketches I, II and III given below show the variation...
Text Solution
|
- A graph plotted between log k versus 1//T for calculating activation e...
Text Solution
|
- 60 g of gaseous C(2)H(6) are mixed with 28 g of carbon monoxide. The p...
Text Solution
|
- How many acidic H - atoms are present in this compound that can react ...
Text Solution
|
- moles of CO(2) evolved during given reaction? What is the value of '...
Text Solution
|
- A current of 2.0A passed for 5 hours through a molten metal salt depos...
Text Solution
|
- If XeOF(4) how many angles are of 90^(@)?
Text Solution
|