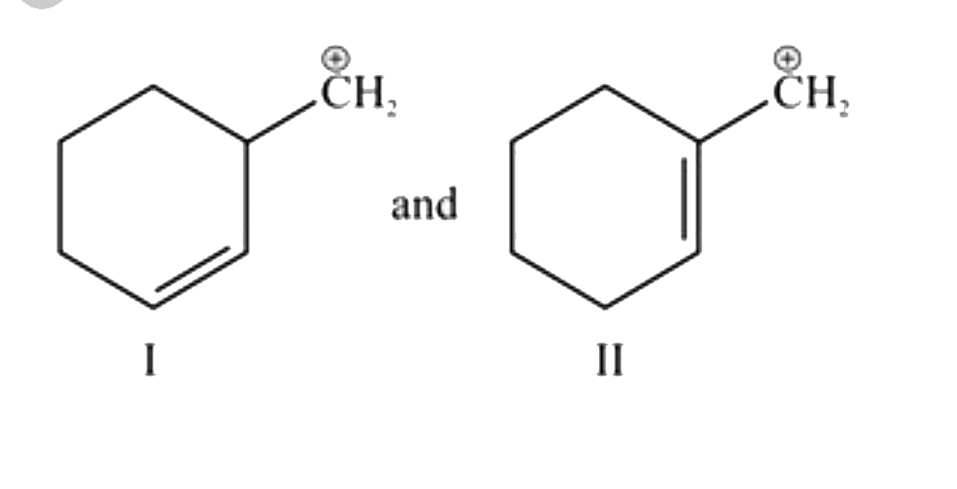
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 97-CHEMISTRY
- The spinel structure consists of an array of O^(2-) ions in fcc arrang...
Text Solution
|
- Exess of KI reacts with CuSO(4) solution and then Na(2)S(2)O(3) soluti...
Text Solution
|
- In each of the following pairs of ions in which I ion is more stable t...
Text Solution
|
- The hybridisation of the central atom will change when
Text Solution
|
- V(1) mL of NaOH of normality X and V(2) mL of Ba(OH)(2) of mormality Y...
Text Solution
|
- If DeltaG^(@)[HI(g)=-1.7kJ], the equilibrium constant for the reaction...
Text Solution
|
- 1 - Bromo -2, 2 - dimethylcyclohexane on treatment with methanol give...
Text Solution
|
- The major product P of the following reaction is
Text Solution
|
- In which of the following compounds hydrolysis tkes plcae through S(N^...
Text Solution
|
- The voltage of the cell consisting of Li(s) and F(2)(g) electrodes is ...
Text Solution
|
- The voltage the characteristics is not common between [Cu(en)(2)]^(2+)...
Text Solution
|
- AgBr(s)+2S(2)O(3)^(2-)(aq)hAq Ag(S(2)O(3))(2)^(3-)(aq)+Br^(-)(aq) Gi...
Text Solution
|
- The incorrect statement regarding above reactions is
Text Solution
|
- In the reaction shown below, identify the correct combination of te in...
Text Solution
|
- For adsorption of a gas on a solid, the plot of log (x//m) vs log P is...
Text Solution
|
- One of the hydrolysed product of the following compound does not react...
Text Solution
|
- In the reaction sequence C(6)H(5)-underset(O)underset(||)C-CH(3)overse...
Text Solution
|
- When A(2) and B(2) are allowed to react, the equilibrium constant of t...
Text Solution
|
- Arrange reactivity of given compounds in decreasing order for hydrolys...
Text Solution
|
- In the reaction x A rarr yB, log{-(d[A])/(dt)}=log{+(d[B])/(dt)}+0.3 T...
Text Solution
|