Similar Questions
Explore conceptually related problems
Recommended Questions
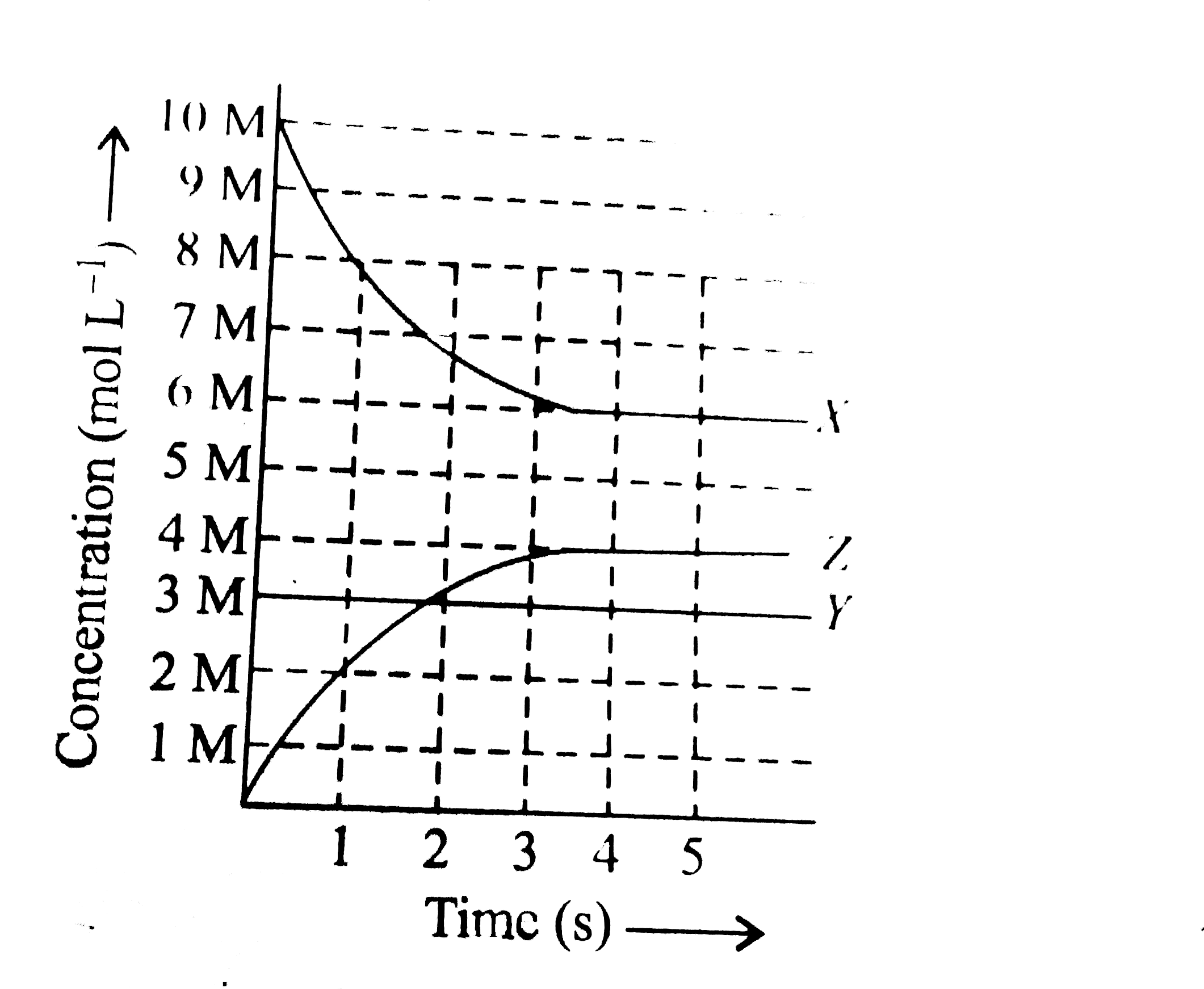
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentratio...
Text Solution
|
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentration ...
Text Solution
|
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentratio...
Text Solution
|
- X, Y and Z react in the 1:1:1 stoichiometric ratio. The concentration ...
Text Solution
|
- The equilibrium concentration of x, y and z are 4, 2 and 2 mol L^(-1) ...
Text Solution
|
- A,B and C react in the 1:1:1 stoichiometric ratio. The concentration o...
Text Solution
|
- A,B and C react in the 1:1:1 stoichiometric ratio. The concentration o...
Text Solution
|
- A 550 K, the K(c) for the following reaction is 10^(4) mol^(-1) L ...
Text Solution
|
- The equilibrium constants for the reactions X = 2Y and Z=P+Q are K(1) ...
Text Solution
|