Text Solution
Verified by Experts
Topper's Solved these Questions
STOICHIOMETRY AND BALANCING REDOX REACTION
FIITJEE|Exercise SOLVED PROBLEMS (OBJECTIVE )|25 VideosSTOICHIOMETRY AND BALANCING REDOX REACTION
FIITJEE|Exercise SINGLE INTEGER TYPE QUESTIONS|2 VideosSTOICHIOMETRY AND BALANCING REDOX REACTION
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|5 VideosSOLID STATE
FIITJEE|Exercise (SINGLE INTEGER ANSWER TYPE QUESTION)|5 VideosTEST PAPERS
FIITJEE|Exercise CHEMISTRY|747 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-STOICHIOMETRY AND BALANCING REDOX REACTION -SOLVED PROBLEMS (SUBJECTIVE )
- 1.20 gm sample of NaCO(3) " and " K(2)CO(3) was dissolved in water to ...
Text Solution
|
- 0.5 g mixture of K(2)Cr(2)O(7) and KMnO(4) was treated with excess of ...
Text Solution
|
- 10 mL of 1.0 M aqueous solution of Br(2) is added to excess of NaOH i...
Text Solution
|
- A sample of hard water contains 96 pp m."of" SO(4)^(2-) and 183 pp m "...
Text Solution
|
- A 2.18 g sample contains a mixture of XO and X(2)O(3). It reacts with ...
Text Solution
|
- One litre of mixture of O2 and O3 at STP was allowed to react with an ...
Text Solution
|
- A 10 g samle of only CuS and Cu(2)S was treated with 100 ml of 1.25 M ...
Text Solution
|
- 30 ml of a solution containing 9.15 gm/litre of an oxalate K(x)H(y) (C...
Text Solution
|
- 12 gm of an impure sample oxide (As(2)O(3) which is acidic in nature ...
Text Solution
|
- An aqueous solution containing 0.10 g KIO(3) (formula weight=214.0) wa...
Text Solution
|
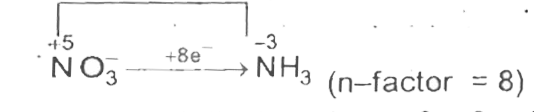
- Mg can reduce NO(3)^(-)" to " NH(3) in basic medium. NO(3)^(-) + Mg ...
Text Solution
|
- For estimating ozone in the air, a certain volume of air is passed thr...
Text Solution
|
- Hydrogen peroxide solution (20 ml) reacts quantitavely with a solution...
Text Solution
|
- 1.0 gm of moist sample of mixture of potassium chlorate (KClO(3)) and ...
Text Solution
|
- Chile salt peter a soure of NaNO(3) also contains NalO(3). The NalO(3)...
Text Solution
|
- 1 gm sample of AgNO(3) is dissolved in 50 ml of water . It is titrated...
Text Solution
|