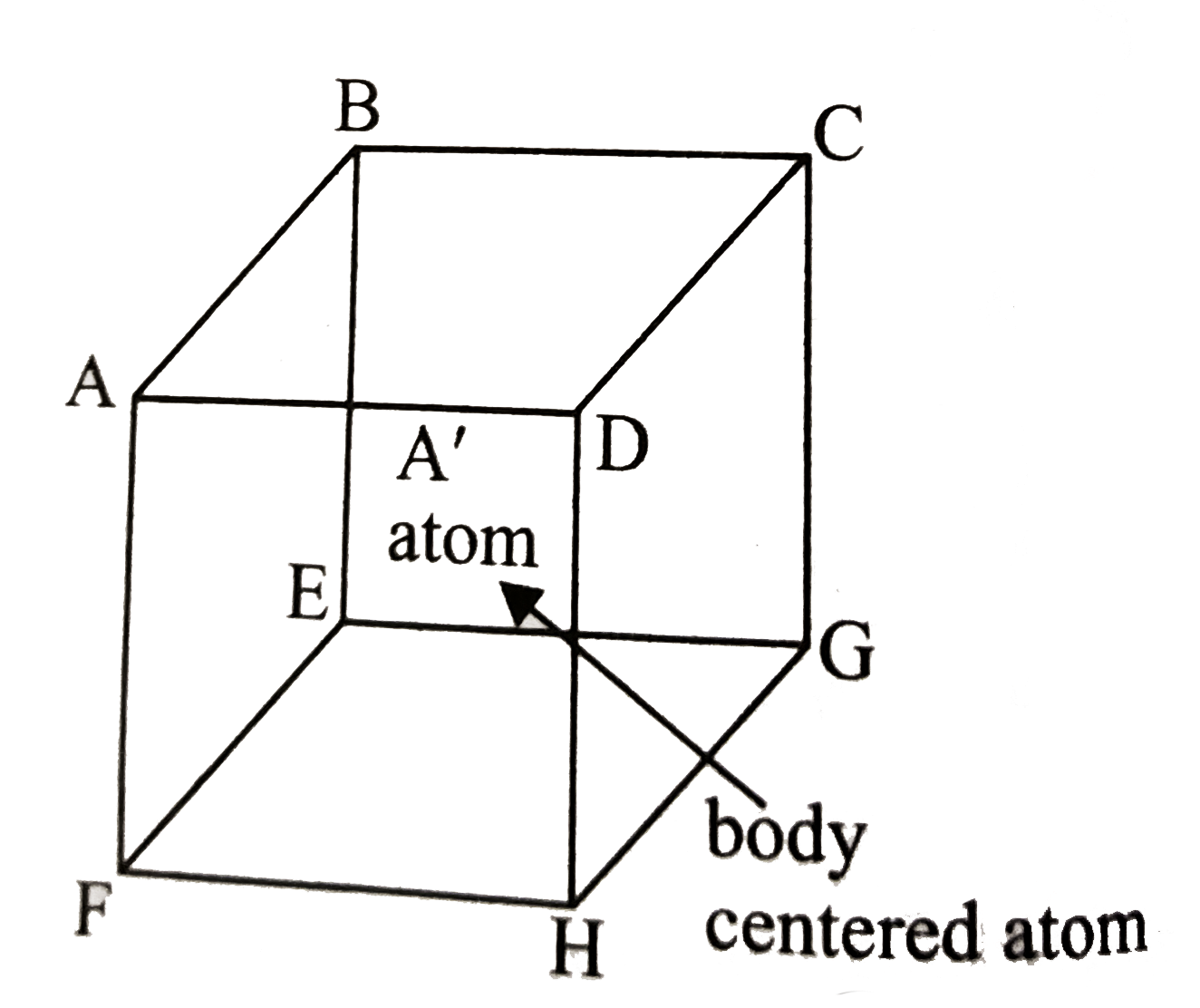
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
SOLID STATE
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) Level - II|25 VideosSOLID STATE
FIITJEE|Exercise (REASONING TYPE QUESTIONS)|1 VideosSOLID STATE
FIITJEE|Exercise ASSIGNMENT PROBLEMS (SUBJECTIVE) Level - II|15 VideosQUALITATIVE ANALYSIS
FIITJEE|Exercise Single interger answer type|3 VideosSTOICHIOMETRY AND BALANCING REDOX REACTION
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|5 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-SOLID STATE-ASSIGNMENT PROBLEMS (OBJECTIVE) Level - I
- The number of tetrahedral and octahedral voids in hexagonal primitive ...
Text Solution
|
- If 'a' be the edge length of the unit cell and r be the radius of an a...
Text Solution
|
- In body-centred cubic lattice given below, the three disntances AB, AC...
Text Solution
|
- A solid is made up of two elements A and B . Atoms B are in ccp arrang...
Text Solution
|
- A binary solid(A^(+) B^(-)) has a zinc blende stracture with B ions co...
Text Solution
|
- In a metal oxide , the oxide ions are arranged in hexagonal close pack...
Text Solution
|
- Schottky defect to crystals is observed when
Text Solution
|
- In a solid AB of NaCl structure, A atoms occupy the corner of the cubi...
Text Solution
|
- The defect when an ion occupies an interstitial position in the crysta...
Text Solution
|
- The coordination number of a metal crystallizing in a hexagonal close-...
Text Solution
|
- In which of the following crystals alternate tetrahedral voids are occ...
Text Solution
|
- In the radii of A^(+) and B^(-) are 95 pm and 181 pm respectively, the...
Text Solution
|
- In a metal M having BCC arrangement edge length of the unit cell is 40...
Text Solution
|
- In a face centred cubic cell, an atom at the face centre is shared by-
Text Solution
|
- KBr shows which of the following defects ?
Text Solution
|
- If in diamond, there is a unit cell of carbon atoms as fcc and if carb...
Text Solution
|
- In which of the following crystals alternate tetrahedral voids are occ...
Text Solution
|
- The ionic radii of Rb^(+) and I^(-) are 1.46 and 2.16Å. The most proba...
Text Solution
|
- Find the molecular formula of a compound made by atoms A and B, where ...
Text Solution
|
- A binary solid A^(+) B^(-) has a structure with B^(-) ions constitutin...
Text Solution
|