Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS AND THERMOCHEMISTRY
FIITJEE|Exercise SOLVED PROBLEM (SUBJECTIVE)|13 VideosTHERMODYNAMICS AND THERMOCHEMISTRY
FIITJEE|Exercise SOLVED PROBLEMS (OBJECTIVE)|28 VideosTEST PAPERS
FIITJEE|Exercise CHEMISTRY|747 VideosTRANSITION ELEMENTS & COORDINATION COMPOUNDS
FIITJEE|Exercise MATCHIG LIST TYPE QUESTIONS|1 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-THERMODYNAMICS AND THERMOCHEMISTRY -SINGLE INTEGER ANSWER TYPE QUESTIONS
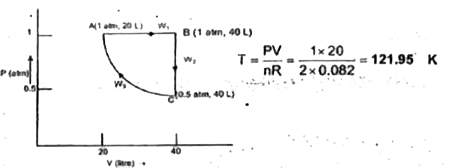
- Two moles of a perfect gas undergo the following process: (a) A revers...
Text Solution
|
- 4.48 L of an ideal gas at STP requires 12 cal to raise its temperature...
Text Solution
|
- What is the change in internal energy when 4 Kj of work is done on the...
Text Solution
|
- Find the value of log(10)K for the reaction A <implies B Given, Delt...
Text Solution
|
- Latent heat of fusion of ice is 6.02 Kj "mol"^(-1). The heat capacity ...
Text Solution
|
- Heat of combustion of diamond =-91 kcal "mol"^(-1) and heat of combust...
Text Solution
|