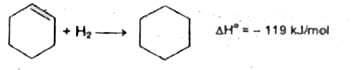
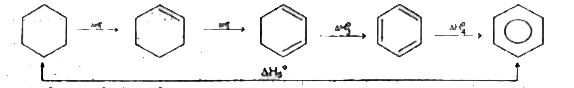
Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS AND THERMOCHEMISTRY
FIITJEE|Exercise SOLVED PROBLEMS (OBJECTIVE)|28 VideosTHERMODYNAMICS AND THERMOCHEMISTRY
FIITJEE|Exercise SOLVED PROBLEMS (OBJECTIVE) (REASONING TYPE QUESTIONS)|4 VideosTHERMODYNAMICS AND THERMOCHEMISTRY
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|5 VideosTEST PAPERS
FIITJEE|Exercise CHEMISTRY|747 VideosTRANSITION ELEMENTS & COORDINATION COMPOUNDS
FIITJEE|Exercise MATCHIG LIST TYPE QUESTIONS|1 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-THERMODYNAMICS AND THERMOCHEMISTRY -SOLVED PROBLEM (SUBJECTIVE)
- One mole of an ideal gas (C(v) = 3/2R) is heated at constant pressure ...
Text Solution
|
- Calculate heat of formation of isoprene using bond energy data. 5C(s...
Text Solution
|
- From the following data, calculate the enthalpy change for the combust...
Text Solution
|
- Calculate the resonance energy of N(2)O from the following data: Del...
Text Solution
|
- 1.0litre sample of a mixture of CH(4) and O(2) measured at 25^(@)C and...
Text Solution
|
- The commercial production of water gas utilizes the reaction under sta...
Text Solution
|
- In order to get maximum calorific output a burner should have an optim...
Text Solution
|
- An athelete is given 100 g glucose of energy equivalenet to 1560 kJ. H...
Text Solution
|
- The standard molar enthalpies of formation of cyclohexane (I) and benz...
Text Solution
|
- Calculate the heat of neutralisation from the following data: 200mL ...
Text Solution
|
- A gas mixture of 3.67L of ethylene and methane on complete combustion ...
Text Solution
|
- Diborane is a potential rocket fuel with undergoes combustion accordin...
Text Solution
|
- a. A cylinder of gas is assumed to contain 11.2 kg of butane. If a nor...
Text Solution
|