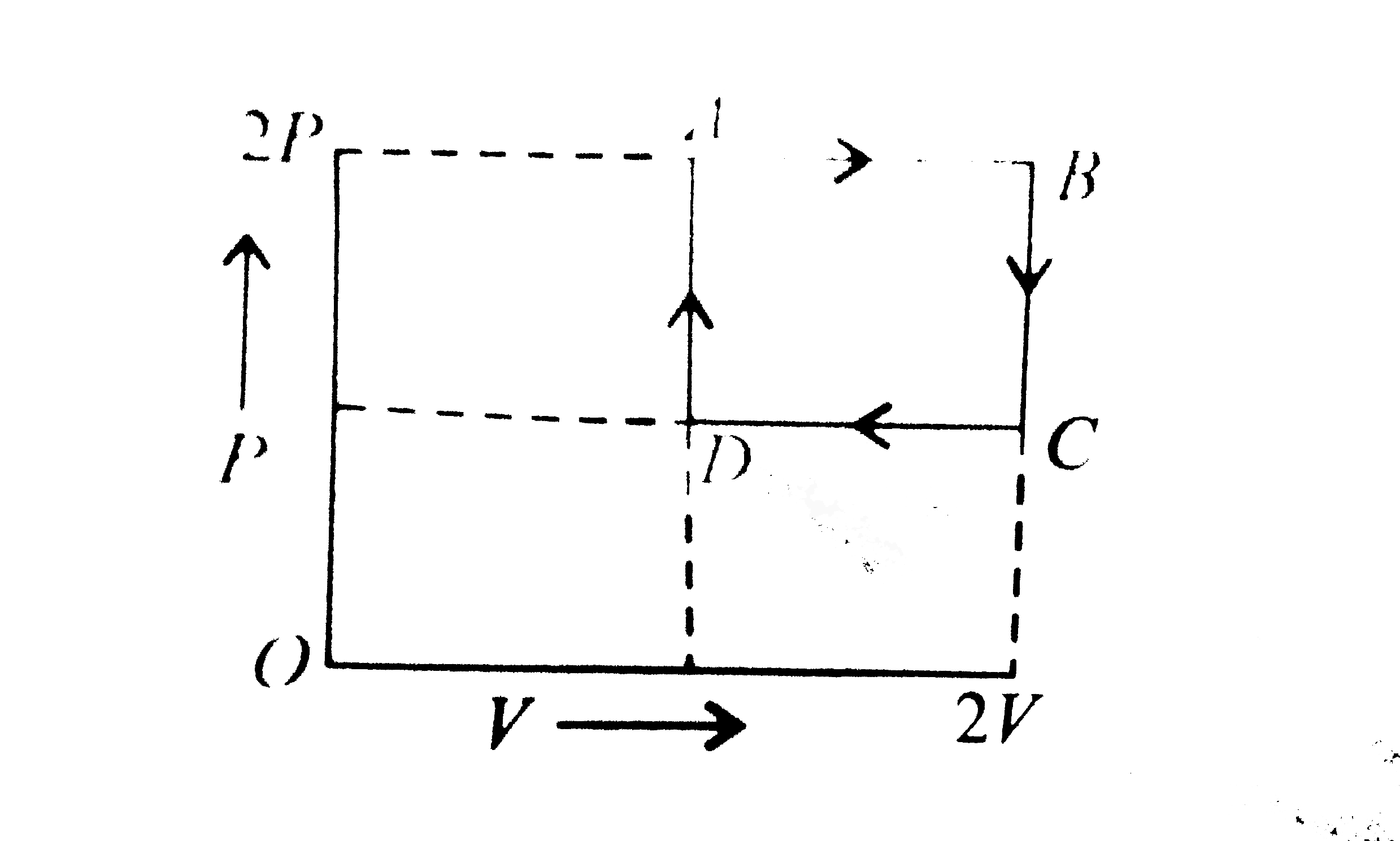
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
THERMODYNAMICS AND THERMOCHEMISTRY
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) COMPREHENSION -II|5 VideosTHERMODYNAMICS AND THERMOCHEMISTRY
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) COMPREHENSION -III|6 VideosTHERMODYNAMICS AND THERMOCHEMISTRY
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) LEVEL-II|25 VideosTEST PAPERS
FIITJEE|Exercise CHEMISTRY|747 VideosTRANSITION ELEMENTS & COORDINATION COMPOUNDS
FIITJEE|Exercise MATCHIG LIST TYPE QUESTIONS|1 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-THERMODYNAMICS AND THERMOCHEMISTRY -ASSIGNMENT PROBLEMS (OBJECTIVE) COMPREHENSION -I
- The state of a mole of an ideal gas changed from state A at pressure 2...
Text Solution
|
- The state of a mole of an ideal gas changed from state A at pressure 2...
Text Solution
|
- The state of a mole of an ideal gas changed from state A at pressure 2...
Text Solution
|
- The state of a mole of an ideal gas changed from state A at pressure 2...
Text Solution
|