A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
LIQUID SOLUTION
FIITJEE|Exercise COMPREHENSION-I|3 VideosLIQUID SOLUTION
FIITJEE|Exercise COMPREHENSION-II|3 VideosLIQUID SOLUTION
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE) level-I|55 VideosIONIC EQUILIBRIUM
FIITJEE|Exercise SINGLE INTEGER ANSWER QUESTIONS|4 VideosNUCLEIC ACID AND VITAMIN
FIITJEE|Exercise ASSIGNMENT PROBLEMS (OBJECTIVE)|15 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-LIQUID SOLUTION -ASSIGNMENT PROBLEMS (OBJECTIVE) level-II
- In the depression of freezing point experiment, it is found that the:
Text Solution
|
- Among 1% solutions of urea. Glucose and sucrose
Text Solution
|
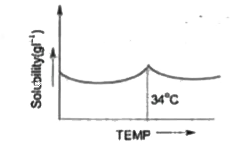
- Solubility curve of Na(2)SO(4).10H(2)O in water with temperature is gi...
Text Solution
|
- On mixing 1 mole of C(6)H(6)(P^(@)=42mm) and 2 mole of C(7)H(8)(P^(@)=...
Text Solution
|
- Which one of the following statemnents is false about osmotic pressure...
Text Solution
|
- At what temperature (s) a 5% solution (w//V) of glucose will develop a...
Text Solution
|
- Which of the following statement is correct?
Text Solution
|
- At the same temperature which of the following solutions will be isoto...
Text Solution
|
- Which one of the following given below concerning properties of solut...
Text Solution
|
- The mole fraction of water in 20% (wt.//wt.) aqueous solution of H(2)O...
Text Solution
|
- The molal freezing point constant for water is 1.86^(@)C//m. Therefore...
Text Solution
|
- Which statement/s is/are correct?
Text Solution
|
- Which of the following is/are not lyophobic in nature?
Text Solution
|
- Which are the properties of sols ?
Text Solution
|
- Migration of colloidal particles under the influence of electrie field...
Text Solution
|
- A finely divided state of the catalyst is more efficient because in th...
Text Solution
|
- Consider the following vapour presure compositon graph. Hence.
Text Solution
|
- K(2)HgI(4) is 50% ionised in aqueous solution. Which of the following ...
Text Solution
|
- Identify the correct statement
Text Solution
|
- Consider following solutions : I: 1M aqueous glucose solution II: 1M...
Text Solution
|