Text Solution
Verified by Experts
Topper's Solved these Questions
TRANSITION ELEMENTS & COORDINATION COMPOUNDS
FIITJEE|Exercise SOLVED PROBLEMS (OBJECTIVE)|48 VideosTRANSITION ELEMENTS & COORDINATION COMPOUNDS
FIITJEE|Exercise COMPREHENSION|6 VideosTRANSITION ELEMENTS & COORDINATION COMPOUNDS
FIITJEE|Exercise MATCHIG LIST TYPE QUESTIONS|1 VideosTHERMODYNAMICS AND THERMOCHEMISTRY
FIITJEE|Exercise SINGLE INTEGER ANSWER TYPE QUESTIONS|5 Videos
Similar Questions
Explore conceptually related problems
FIITJEE-TRANSITION ELEMENTS & COORDINATION COMPOUNDS-SOLVED PROBLEMS(SUBJECTIVE)
- Fe^(3+)overset(SCN^(-)(excess))to "blood red"(A)overset(F^(-)(excess))...
Text Solution
|
- A(1) & A(2) are two ores of metal M. Ar on calcination gives black pre...
Text Solution
|
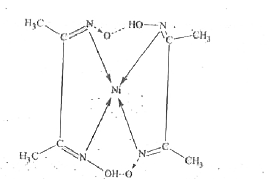
- Nickel chloride, when treated with dimethyl gyloxime in presence of am...
Text Solution
|
- Write the IUPAC nomenclature of the given complex along with its hybri...
Text Solution
|
- Deduce the structures of [NiCl(4)]^(2-) and [Ni (CN)(4)]^(2-) consider...
Text Solution
|
- A metal complex having composition Cr(NH(3))(4)CI(2) Br has been isola...
Text Solution
|
- Draw the structures of [Co(NH(3))(6)]^(3+) [Ni(CN)(4)]^(2-) and [Ni(CO...
Text Solution
|
- The magnetic moment of [Mn(CN)(6)]^(3-) is 2.8 B.M and that of [MnBr(4...
Text Solution
|
- Why chelated complexes are more stable than unchelated complexes
Text Solution
|
- The hexaaqua complex[Ni(H(2)O)(6)]^(2+) is green, whereas the correspo...
Text Solution
|
- Calculate magnetic moment of Fe^(3+) in [Fe(CN)(6)]^(3+) and in [Fe(H(...
Text Solution
|
- Give the formula of three ions which are coloured due to charge transf...
Text Solution
|
- Classify each of the following complexes as either high or low spin. E...
Text Solution
|
- How do you account for the indicated molecular geometry for the follow...
Text Solution
|
- An aqueous solution of CoCl(3).6NH(3) is found to be a better conducto...
Text Solution
|
- Account for the following: Co(ll) is stable in aqueous solution but in...
Text Solution
|
- The compounds of Zn, Cd and Hg are usually white. (ii) A dark blue pr...
Text Solution
|
- A, B and C are three complexes of chromium(III) with empirical formula...
Text Solution
|
- [NiC(4)]^(2-)It is paramagnetic while Ni[CO(4)] is diamagnetic though ...
Text Solution
|
- Identify the complexes expected to be coloured and explain Ti(NO(3))(4...
Text Solution
|