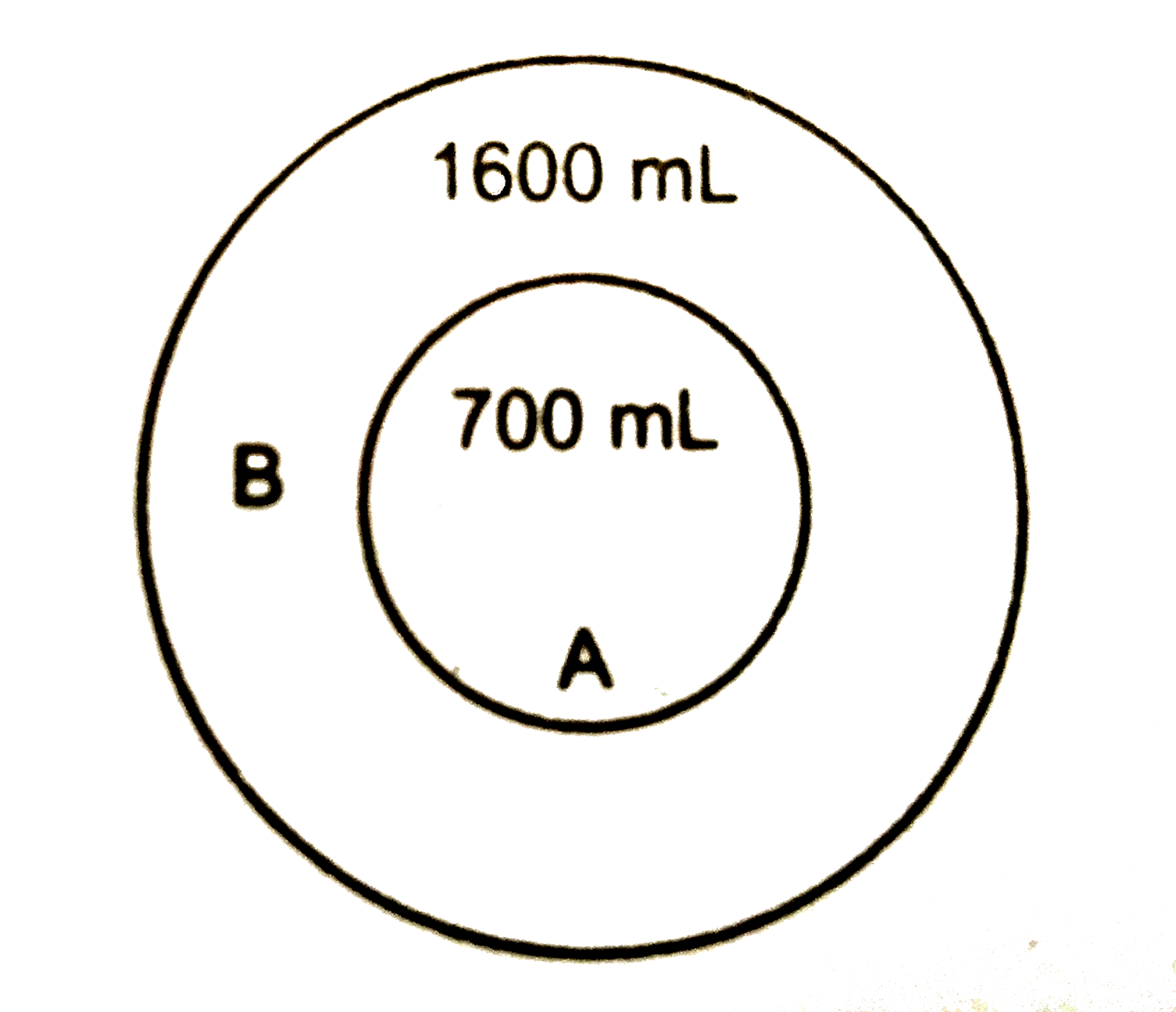Similar Questions
Explore conceptually related problems
Recommended Questions
- Two balloon A and B are taken at 300 K. Maximum capacity of balloon A ...
Text Solution
|
- A bolloon blown up has a volume of 300 mL at 27% C. The balloon is dis...
Text Solution
|
- Two inflated ballons I and II (thin skin) having volume 600 mL and 150...
Text Solution
|
- Two balloon A and B are taken at 300 K. Maximum capacity of balloon A ...
Text Solution
|
- फूले हुए गुब्बारे को गर्म करने पर वह क्यों फूट जाता है?
Text Solution
|
- A toy balloon blown up at 5^(@)C has a volume of 480 mL. At this stage...
Text Solution
|
- A balloon is filled with one mole of helium at 0^@C and 1 bar pressure...
Text Solution
|
- Two inflated ballons I and II (thin skin) having volume 600 mL and 150...
Text Solution
|
- Bursting of balloon is a change.
Text Solution
|