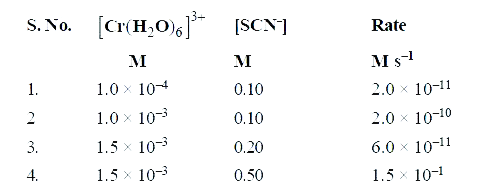Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The reaction [Cr(H2O)6]^(3+)(aq)+ SCN^(-) (aq) to [Cr(H2O)5NCS]^(2+) (...
Text Solution
|
- H2CO3(aq)+H2O(l)toHCO3^-(aq) + H3O^+(aq) HCO3^(-)(aq) + H2O(l)to CO3^(...
Text Solution
|
- For ortho phosphoric acid, H3PO4(aq)+H2O(aq) to H3O^(+)(aq)+H2PO4^(-)(...
Text Solution
|
- what is the standard electrode potential for the reduction of HClO? ...
Text Solution
|
- Balance the following equation stepwise. NaOH(aq) + H2SO4(aq) to Na2SO...
Text Solution
|
- The reaction [Cr(H2O)6]^(3+)(aq)+ SCN^(-) (aq) to [Cr(H2O)5NCS]^(2+) (...
Text Solution
|
- The reaction [Cr(H2O)6]^(3+)(aq)+ SCN^(-) (aq) to [Cr(H2O)5NCS]^(2+) (...
Text Solution
|
- The reaction [Cr(H2O)6]^(3+)(aq)+ SCN^(-) (aq) to [Cr(H2O)5NCS]^(2+) (...
Text Solution
|
- Equivalent to the following reactions Kc - Write the lines. CH3COOC2H5...
Text Solution
|